Bryostatin 3Potent protein kinase C activator CAS# 143370-84-7 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- BET-BAY 002
Catalog No.:BCC5510
CAS No.:1588521-78-1
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
Quality Control & MSDS
Number of papers citing our products

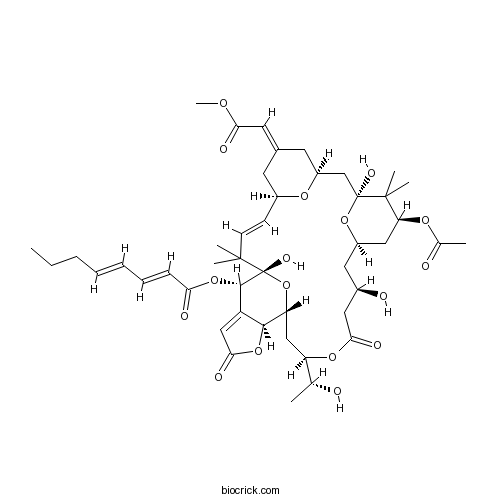
Chemical structure
3D structure
| Cas No. | 143370-84-7 | SDF | Download SDF |
| PubChem ID | 10395929 | Appearance | Powder |
| Formula | C46H64O17 | M.Wt | 888.99 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| SMILES | CCCC=CC=CC(=O)OC1C2=CC(=O)OC2C3CC(OC(=O)CC(CC4CC(C(C(O4)(CC5CC(=CC(=O)OC)CC(O5)C=CC(C1(O3)O)(C)C)O)(C)C)OC(=O)C)O)C(C)O | ||
| Standard InChIKey | BSNHYLUEHJOXFN-HBUIWOJHSA-N | ||
| Standard InChI | InChI=1S/C46H64O17/c1-9-10-11-12-13-14-37(50)61-42-33-23-40(53)60-41(33)35-24-34(26(2)47)59-39(52)21-29(49)20-31-22-36(57-27(3)48)44(6,7)45(54,62-31)25-32-18-28(19-38(51)56-8)17-30(58-32)15-16-43(4,5)46(42,55)63-35/h11-16,19,23,26,29-32,34-36,41-42,47,49,54-55H,9-10,17-18,20-22,24-25H2,1-8H3/b12-11+,14-13+,16-15+,28-19+/t26-,29-,30+,31-,32+,34-,35-,36+,41+,42+,45+,46-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent protein kinase C activator (KI = 2.75 nM). Attenuates phorbol 12-myristate 13-acetate inhibition of GH4C1 pituitary cell proliferation in vitro. |

Bryostatin 3 Dilution Calculator

Bryostatin 3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1249 mL | 5.6244 mL | 11.2487 mL | 22.4974 mL | 28.1218 mL |
| 5 mM | 0.225 mL | 1.1249 mL | 2.2497 mL | 4.4995 mL | 5.6244 mL |
| 10 mM | 0.1125 mL | 0.5624 mL | 1.1249 mL | 2.2497 mL | 2.8122 mL |
| 50 mM | 0.0225 mL | 0.1125 mL | 0.225 mL | 0.4499 mL | 0.5624 mL |
| 100 mM | 0.0112 mL | 0.0562 mL | 0.1125 mL | 0.225 mL | 0.2812 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SMIP004
Catalog No.:BCC1955
CAS No.:143360-00-3
- RS 56812 hydrochloride
Catalog No.:BCC6877
CAS No.:143339-12-2
- UNC 2400
Catalog No.:BCC5625
CAS No.:1433200-49-7
- Ac-YVAD-CHO
Catalog No.:BCC4021
CAS No.:143313-51-3
- H-Chg-OH
Catalog No.:BCC3162
CAS No.:14328-51-9
- RA-XI
Catalog No.:BCN3514
CAS No.:143277-27-4
- AGI-6780
Catalog No.:BCC1331
CAS No.:1432660-47-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- Tin protoporphyrin IX dichloride
Catalog No.:BCC6776
CAS No.:14325-05-4
- Microstegiol
Catalog No.:BCN3157
CAS No.:143246-41-7
- (-)-Isolariciresinol 9'-O-glucoside
Catalog No.:BCN7708
CAS No.:143236-04-8
- (-)-Lyoniresinol 9'-O-glucoside
Catalog No.:BCN7037
CAS No.:143236-02-6
- Naratriptan
Catalog No.:BCC5053
CAS No.:143388-64-1
- SAR131675
Catalog No.:BCC5097
CAS No.:1433953-83-3
- (Arg)9 peptide
Catalog No.:BCC5336
CAS No.:143413-47-2
- Poriol
Catalog No.:BCN6816
CAS No.:14348-16-4
- Cnidilin
Catalog No.:BCN2731
CAS No.:14348-22-2
- 5,8-Dihydroxypsoralen
Catalog No.:BCC8104
CAS No.:14348-23-3
- LOE 908 hydrochloride
Catalog No.:BCC7327
CAS No.:143482-60-4
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Dammarenediol II
Catalog No.:BCN6240
CAS No.:14351-29-2
- 3-Acetoxy-24-hydroxydammara-20,25-diene
Catalog No.:BCN1569
CAS No.:143519-04-4
- (4S,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic acid
Catalog No.:BCN8364
CAS No.:143527-70-2
- Shancigusin I
Catalog No.:BCN8272
CAS No.:1435488-35-9
Bryostatin-1, Fenretinide and 1alpha,25 (OH)(2)D(3) Induce Growth Inhibition, Apoptosis and Differentiation in T and B Cell-Derived Acute Lymphoblastic Leukemia Cell Lines (CCRF-CEM and Nalm-6).[Pubmed:23407583]
Avicenna J Med Biotechnol. 2011 Oct;3(4):177-93.
In many acute leukemias, normal differentiation does not occur. However, in many cell lines derived from hematologic malignancies, differentiation or apoptosis can be induced by variety of agents. Despite advances in the treatment of Acute Lymphoblastic Leukemia (ALL), in most patients long-term survival rates remain unsatisfactory, especially in T-cell derived ALL. Thus we studied the anti-cancer effects of fenretinide, 1alpha,25(OH)(2)D(3), and bryostatin-1 in CCRF-CEM (T-cell derived) and Nalm-6 (B-cell derived) ALL cell lines. Using MTT assays, both cell lines were shown to exhibit increased inhibition of proliferation at micro (fenretinide) and nanomolar (1alpha,25(OH)(2)D(3), bryostatin-1) concentrations. These anti-cancer agents were shown to induce apoptosis and activate caspase-3 pathway in both ALL cell lines. Furthermore, for the first time we are reporting consistent anti-proliferative and apoptotic effects of Bryostatin-1 in ALL T-cell derived cell line with the lowest ED(50) (ranging 4.6-7.4 nM). To evaluate the differentiation induction by fenretinide, 1alpha,25(OH)(2)D(3), and bryostatin-1 in ALL cell lines, we assayed for the expressions of CD19, CD38 markers on Nalm-6 and CD7 marker on CCRF-CEM cell line. The flow cytometric analysis showed a significant increase in expression of CD markers in response to anti-cancer drug treatments. To assay the effects of anti-cancer drugs on cell cycle distribution, cell cycle analysis using flow cytometry was employed. These anti-cancer drugs appear to affect the CCRF-CEM and Nalm-6 cell cycles differently (G0/G1 and G2/M arrest, respectively). Overall results demonstrate that the anti-cancer agents used in this study are strong inhibitors of ALL cell proliferation and inducers of apoptosis and differentiation in vitro. These findings may be quite helpful if these drugs are to be used for differentiation therapy of ALL patients in clinics in the future. Further studies are warranted to establish the in vivo effect of these drugs particularly in patients with T-cell derived ALL.
Reversible phosphorylation of Bcl2 following interleukin 3 or bryostatin 1 is mediated by direct interaction with protein phosphatase 2A.[Pubmed:9852076]
J Biol Chem. 1998 Dec 18;273(51):34157-63.
Interleukin 3 (IL-3) stimulates the net growth of murine factor-dependent NSF/N1.H7 and FDC-P1/ER myeloid cells by stimulating proliferation and suppressing apoptosis. Recently, we discovered that Bcl2 is phosphorylated at an evolutionarily conserved serine residue (Ser70) after treatment with the survival agonists IL-3 or bryostatin 1, a potent activator of protein kinase (Ito, T., Deng, X., Carr, B., and May, W. S. (1997) J. Biol. Chem. 272, 11671-11673). In addition, an intact Ser70 was found to be required for Bcl2's ability to suppress apoptosis after IL-3 withdrawal or toxic chemotherapy. We now show that phosphorylation of Bcl2 occurs rapidly after the addition of agonist to IL-3-deprived cells and can be reversed by the action of an okadaic acid (OA)-sensitive phosphatase. A role for protein phosphatase (PP) 2A as the Bcl2 regulatory phosphatase is supported by several observations: 1) dephosphorylation of Bcl2 is blocked by OA, a potent PP1 and PP2A inhibitor; 2) intracellular PP2A, but not PP1, co-localizes with Bcl2; 3) the purified PP2Ac catalytic subunit directly dephosphorylates Bcl2 in vitro in an OA-sensitive manner; 4) the purified PP2Ac catalytic subunit preferentially dephosphorylates Bcl2 in vitro compared with PP1 and PP2B; 5) reciprocal immunoprecipitation studies indicate a direct interaction between PP2A and hemagglutinin (HA)-Bcl2; and 6) treatment of factor-deprived cells with bryostatin 1 dramatically increases the association between PP2A and Bcl2. Increased association between Bcl2 and PP2A occurs 15 min after agonist stimulation when Bcl2 phosphorylation has peaked and immediately before dephosphorylation. An agonist-induced increased association of PP2A and Bcl2 fails to occur in cells expressing the inactive, phosphorylation-negative S70A Bcl2 mutant, which indicates that an intact Ser70 site is necessary and sufficient for the interaction to occur. Functional phosphorylation of Bcl2 at Ser70 is proposed to be a dynamic process regulated by the sequential action of an agonist-activated Bcl2 kinase and PP2A.
Enantio- and diastereoselective additions to aldehydes using the bifunctional reagent 2-(chloromethyl)-3-(tributylstannyl)propene: application to a synthesis of the C16-C27 segment of bryostatin 1.[Pubmed:15787541]
J Org Chem. 2005 Apr 1;70(7):2543-50.
[reaction: see text] Reactions of the bifunctional allylstannane 2-(chloromethyl)-3-(tributylstannyl)propene with aldehydes have been examined. These generally occur in high yields using Lewis acid promoters and the products can be isolated and purified without incident. Good yields and high enantioselectivities are also realized in catalytic asymmetric allylations (CAA reactions) using the previously described BITIP catalyst system. Protection of the free hydroxyl can be accomplished without cyclization to the derived tetrahydrofuran, although this transformation is also facile. The utility of the incorporated allyl chloride functionality allows for the obvious use of such products in reactions with nucleophiles. Use of these products in a less obvious connective strategy is demonstrated in the synthesis of the C12-C27 segment of bryostatin 1 where a connective, or "lynchpin", double-allylation process was employed. The beta-hydroxy allyl chloride obtained from an initial chelation-controlled allylation of aldehyde 16 was converted to allylstannane 19 and applied in a second allylation reaction, thus allowing for a highly convergent synthesis of the bryostatin C ring backbone in a stereoselective fashion.
Bryostatins selectively regulate protein kinase C-mediated effects on GH4 cell proliferation.[Pubmed:1904062]
J Biol Chem. 1991 Jun 15;266(17):11205-12.
The phorbol ester tumor promoter, 12-O-tetradecanoylphorbol-13-acetate [TPA) or phorbol 12-myristate 13-acetate), directly activates the calcium- and phospholipid-dependent protein kinase C (protein kinase C), which, in turn, generates a number of cellular responses. The bryostatins, a family of macrocyclic lactones isolated from marine bryozoans, also bind to and active protein kinase C. However, they differ from TPA in the selectivity of their responses in that they behave either as agonists or antagonists of protein kinase C actions. We used several bryostatins and TPA to examine the role of protein kinase C in the regulation of GH4C1 rat pituitary tumor cell proliferation. TPA inhibited [3H]thymidine incorporation in GH4 cells in a stereoselective and concentration-dependent manner. Examination of cell cycle distribution by flow cytometry revealed that TPA decreased the percentage of cells in S-phase and proportionally increased the percentage of G1-phase cells. Bryostatin 1 alone did not affect cell proliferation, but prevented the TPA inhibition of cell proliferation. Bryostatin 1 treatment from 30 min to 6 h after TPA treatment also prevented the growth-inhibitory action of TPA, suggesting that prolonged stimulation of protein kinase C is necessary for growth inhibition. Both bryostatin 1 and TPA down-regulated protein kinase C, indicating that down regulation of the enzyme cannot account for the growth inhibitory action of TPA. Bryostatin 2, which differs from bryostatin 1 by a hydroxyl substitution for the acetyl group at the C-7 carbon of the macrocyclic lactone ring (R1), inhibited cell proliferation and did not reduce the growth-inhibitory action of TPA. Bryostatins 3 and 8 (each of which has an ester group in the R1 position, yet contains other structural modifications) are antagonists for TPA inhibition of GH4 cell proliferation like bryostatin 1. We next examined the effect of bryostatins 3 and 8 on cell-substratum adhesion, a cellular response observed after GH4 cells are treated with growth-inhibitory agents. Bryostatin 8 (like bryostatin 1) did not enhance cell-substratum adhesion and blocked the action of TPA. In contrast, Bryostatin 3 enhanced cell-substratum adhesion. Because Bryostatin 3 blocked TPA inhibition of cell proliferation, yet did not block TPA-enhanced cell-substratum adhesion, these responses are not interdependent. We next examined the effect of bryostatin on other growth-inhibitory agents for GH4 cells. Bryostatin 8 blocks the effect of TPA on [3H]thymidine incorporation and the entry of G1 cells into S-phase, but does not block the growth-inhibitory action of thyrotropin-releasing hormone or epidermal growth factor.(ABSTRACT TRUNCATED AT 400 WORDS)
Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C.[Pubmed:3174627]
Proc Natl Acad Sci U S A. 1988 Oct;85(19):7197-201.
The bryostatins are macrocyclic lactones that represent an additional structural class of potent activators of protein kinase C. These marine animal biosynthetic products are of unusual interest because they induce only a subset of the biological responses induced by the phorbol esters. We have now determined the binding affinities of naturally occurring and semisynthetic bryostatins for protein kinase C by competition analysis with [26-3H]bryostatin 4 as the radioactive ligand. Esterification of the hydroxyl group at C26 caused dramatic loss of activity as did inversion of the asymmetric center at this position. In contrast, neither of the ester groups at C7 and C20 had a major influence on activity. Computer modeling of the phorbol esters, related diterpenes, and indole alkaloids suggested that the C20, C9, and C4 oxygens of phorbol represented critical elements of the phorbol ester pharmacophore. The C26 oxygen of the bryostatins, together with the C1 and C19 oxygens, gave an excellent spatial correlation with this model, with a root-mean-square deviation of 0.16 A (compared to 0.10-0.35 A among phorbol-related diterpenes). The extension of the phorbol ester pharmacophore model to the bryostatins and its agreement with the structure-activity relations for the bryostatin class of compounds provide additional support for the validity of the model.


