Bryostatin 2Protein kinase C activator CAS# 87745-28-6 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- BET-BAY 002
Catalog No.:BCC5510
CAS No.:1588521-78-1
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
Quality Control & MSDS
Number of papers citing our products

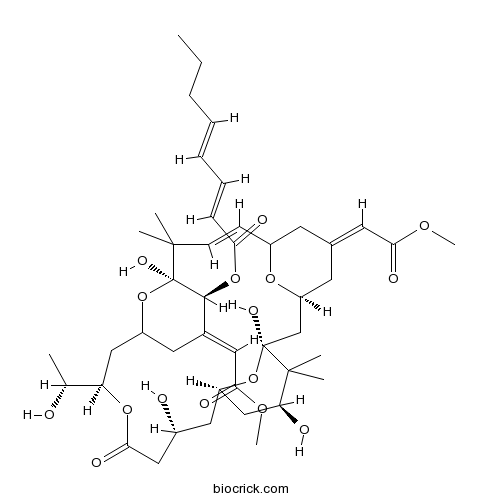
Chemical structure
3D structure
| Cas No. | 87745-28-6 | SDF | Download SDF |
| PubChem ID | 6326659 | Appearance | Powder |
| Formula | C45H66O16 | M.Wt | 863 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| SMILES | CCCC=CC=CC(=O)OC1C(=CC(=O)OC)CC2CC(OC(=O)CC(CC3CC(C(C(O3)(CC4CC(=CC(=O)OC)CC(O4)C=CC(C1(O2)O)(C)C)O)(C)C)O)O)C(C)O | ||
| Standard InChIKey | LIPGUSBNMQRYNL-WZYRAJGLSA-N | ||
| Standard InChI | InChI=1S/C45H66O16/c1-9-10-11-12-13-14-37(49)59-41-29(21-39(51)56-8)20-32-24-35(27(2)46)58-40(52)23-30(47)22-33-25-36(48)43(5,6)44(53,60-33)26-34-18-28(19-38(50)55-7)17-31(57-34)15-16-42(3,4)45(41,54)61-32/h11-16,19,21,27,30-36,41,46-48,53-54H,9-10,17-18,20,22-26H2,1-8H3/b12-11+,14-13+,16-15+,28-19-,29-21+/t27-,30-,31?,32?,33-,34+,35-,36+,41+,44+,45-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protein kinase C (PKC) activator; analog of Bryostatin 1. Attenuates cell proliferation and cell attachment in U937 cells. Inhibits DNA synthesis at 100 nM in SH-SY5Y human neuroblastoma cells. Also inhibits EGF binding and induces arachidonic acid release in vitro. Antitumor. |

Bryostatin 2 Dilution Calculator

Bryostatin 2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1587 mL | 5.7937 mL | 11.5875 mL | 23.175 mL | 28.9687 mL |
| 5 mM | 0.2317 mL | 1.1587 mL | 2.3175 mL | 4.635 mL | 5.7937 mL |
| 10 mM | 0.1159 mL | 0.5794 mL | 1.1587 mL | 2.3175 mL | 2.8969 mL |
| 50 mM | 0.0232 mL | 0.1159 mL | 0.2317 mL | 0.4635 mL | 0.5794 mL |
| 100 mM | 0.0116 mL | 0.0579 mL | 0.1159 mL | 0.2317 mL | 0.2897 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Tyrosinol
Catalog No.:BCC2697
CAS No.:87745-27-5
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
- GPBAR-A
Catalog No.:BCC6201
CAS No.:877052-79-4
- Alismoxide
Catalog No.:BCN1265
CAS No.:87701-68-6
- 3-(1-Piperazinyl)-1,2-benzisothiazole
Catalog No.:BCC8585
CAS No.:87691-87-0
- Isomagnolol
Catalog No.:BCN8325
CAS No.:87688-90-2
- Pentoxyresorufin
Catalog No.:BCC6297
CAS No.:87687-03-4
- 6-Hydroxyrubiadin
Catalog No.:BCN4425
CAS No.:87686-86-0
- Trandolapril
Catalog No.:BCC5275
CAS No.:87679-37-6
- RO-9187
Catalog No.:BCC1904
CAS No.:876708-03-1
- RF 9
Catalog No.:BCC7744
CAS No.:876310-60-0
- Montixanthone
Catalog No.:BCN8069
CAS No.:876305-36-1
- Tandospirone
Catalog No.:BCC4208
CAS No.:87760-53-0
- ML 221
Catalog No.:BCC6278
CAS No.:877636-42-5
- Eupalinolide B
Catalog No.:BCN2525
CAS No.:877822-40-7
- Eupalinolide A
Catalog No.:BCN2524
CAS No.:877822-41-8
- erythro-Guaiacylglycerol beta-sinapyl ether
Catalog No.:BCN6605
CAS No.:877875-96-2
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
- 6beta-Hydroxyipolamiide
Catalog No.:BCN4426
CAS No.:87797-84-0
- Glucagon-like peptide 1 (1-37) (human, rat)
Catalog No.:BCC5827
CAS No.:87805-34-3
- S1RA
Catalog No.:BCC4189
CAS No.:878141-96-9
- Alismol
Catalog No.:BCN4427
CAS No.:87827-55-2
- Walrycin B
Catalog No.:BCC5156
CAS No.:878419-78-4
- Isosalviamine A
Catalog No.:BCN3553
CAS No.:878475-29-7
Bryostatin-1, Fenretinide and 1alpha,25 (OH)(2)D(3) Induce Growth Inhibition, Apoptosis and Differentiation in T and B Cell-Derived Acute Lymphoblastic Leukemia Cell Lines (CCRF-CEM and Nalm-6).[Pubmed:23407583]
Avicenna J Med Biotechnol. 2011 Oct;3(4):177-93.
In many acute leukemias, normal differentiation does not occur. However, in many cell lines derived from hematologic malignancies, differentiation or apoptosis can be induced by variety of agents. Despite advances in the treatment of Acute Lymphoblastic Leukemia (ALL), in most patients long-term survival rates remain unsatisfactory, especially in T-cell derived ALL. Thus we studied the anti-cancer effects of fenretinide, 1alpha,25(OH)(2)D(3), and bryostatin-1 in CCRF-CEM (T-cell derived) and Nalm-6 (B-cell derived) ALL cell lines. Using MTT assays, both cell lines were shown to exhibit increased inhibition of proliferation at micro (fenretinide) and nanomolar (1alpha,25(OH)(2)D(3), bryostatin-1) concentrations. These anti-cancer agents were shown to induce apoptosis and activate caspase-3 pathway in both ALL cell lines. Furthermore, for the first time we are reporting consistent anti-proliferative and apoptotic effects of Bryostatin-1 in ALL T-cell derived cell line with the lowest ED(50) (ranging 4.6-7.4 nM). To evaluate the differentiation induction by fenretinide, 1alpha,25(OH)(2)D(3), and bryostatin-1 in ALL cell lines, we assayed for the expressions of CD19, CD38 markers on Nalm-6 and CD7 marker on CCRF-CEM cell line. The flow cytometric analysis showed a significant increase in expression of CD markers in response to anti-cancer drug treatments. To assay the effects of anti-cancer drugs on cell cycle distribution, cell cycle analysis using flow cytometry was employed. These anti-cancer drugs appear to affect the CCRF-CEM and Nalm-6 cell cycles differently (G0/G1 and G2/M arrest, respectively). Overall results demonstrate that the anti-cancer agents used in this study are strong inhibitors of ALL cell proliferation and inducers of apoptosis and differentiation in vitro. These findings may be quite helpful if these drugs are to be used for differentiation therapy of ALL patients in clinics in the future. Further studies are warranted to establish the in vivo effect of these drugs particularly in patients with T-cell derived ALL.
Mechanistic and computational studies of exocyclic stereocontrol in the synthesis of bryostatin-like cis-2,6-disubstituted 4-alkylidenetetrahydropyrans by Prins cyclization.[Pubmed:23121542]
J Org Chem. 2013 Jan 4;78(1):104-15.
The Prins cyclization of syn-beta-hydroxy allylsilanes and aldehydes gives cis-2,6-disubstituted 4-alkylidenetetrahydropyrans as sole products in excellent yields regardless of the aldehyde (R'') or syn-beta-hydroxy allylsilane substituent (R') used. By reversing the R'' and R' groups, complementary exocyclic stereocontrol can be achieved. When the anti-beta-hydroxy allylsilanes are used, the Prins cyclization gives predominantly cis-2,6-disubstituted 4-alkylidenetetrahydropyrans, now with the opposite olefin geometry in excellent yield. The proposed reaction mechanism and the observed stereoselectivity for these processes are supported by DFT calculations.
Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2.[Pubmed:19198835]
Cancer Immunol Immunother. 2009 Oct;58(10):1565-76.
Regression of established tumors can be induced by adoptive immunotherapy (AIT) with tumor draining lymph node (DLN) lymphocytes activated with bryostatin and ionomycin (B/I). We hypothesized that B/I-activated T cells cultured in IL-7 + IL-15 might proliferate and survive in culture better than cells cultured in IL-2, and that these cells would have equal or greater anti-tumor activity in vivo. Tumor antigen-sensitized DLN lymphocytes from either wild-type or T cell receptor transgenic mice were harvested, activated with B/I, and expanded in culture with either IL-2, IL-7 + IL-15 or a regimen of alternating cytokines. Cell yields, proliferation, apoptosis, phenotypes, and in vitro responses to tumor antigen were compared for cells grown in different cytokines. These T cells were also tested for anti-tumor activity against melanoma lung metastases established by prior i.v. injection of B16 melanoma cells. IL-7 + IL-15 or alternating cytokines resulted in much faster and prolonged proliferation and much less apoptosis of B/I-activated T cells than culturing the same cells in IL-2. This resulted in approximately tenfold greater yields of viable cells. Culture in IL-7 + IL-15 yielded higher proportions of CD8+ T cells and a higher proportion of cells with a central memory phenotype. Despite this, T cells grown in IL-7 + IL-15 had higher IFN-gamma release responses to tumor antigen than cells grown in IL-2. Adoptive transfer of B/I-activated T cells grown in IL-7 + IL-15 or the alternating regimen had equal or greater efficacy on a "per-cell" basis against melanoma metastases. Activation of tumor antigen-sensitized T cells with B/I and culture in IL-7 + IL-15 is a promising modification of standard regimens for production of T cells for use in adoptive immunotherapy of cancer.
A randomized phase II trial of interleukin-2 in combination with four different doses of bryostatin-1 in patients with renal cell carcinoma.[Pubmed:16514482]
Invest New Drugs. 2006 Mar;24(2):141-9.
PURPOSE: Bryostatin-1 is a PKC modulator with direct anti-tumor activity and immunomodulatory properties. We combined different doses of Bryostatin-1 with IL-2 to determine effects on clinical response rate and T cell phenotype in patients with advanced kidney cancer. EXPERIMENTAL DESIGN: IL-2 naive patients were given 11 x 10(6) IU subcutaneously of IL-2 on days 1-4, 8-11, and 15-18 of every 28-day cycle. Twenty four patients were randomized to treatment cohorts of 5, 15 or 25 mcg/m2 of Bryostatin-1 on days 1, 8 and 15, starting in the second cycle. An additional nine, non-randomized patients were given 35 mcg/m2. Lymphocytes were analyzed for number, activation status, and production of IL-2, IL-4 and IFN-gamma. Response evaluation was performed every 3 cycles. RESULTS: Common grade 3 toxicities included fatigue (5), nausea/vomiting (5), myopathy (3), dyspnea (3), and syncope (3). Four patients, in the two highest dose cohorts, demonstrated evidence of tumor shrinkage, although there was only 1 objective PR. The median time to progression was 104 days (95% CI 88-120) and the median survival was 452 days (95% CI = 424-480). There was no significant boosting effect of Bryostatin-1 on lymphocytes. CONCLUSIONS: The addition of Bryostatin-1 to IL-2 was well tolerated, but the overall response rate was low (3.2%), indicating that further studies with this combination are not warranted.
Comparison of transcriptional response to phorbol ester, bryostatin 1, and bryostatin analogs in LNCaP and U937 cancer cell lines provides insight into their differential mechanism of action.[Pubmed:23146662]
Biochem Pharmacol. 2013 Feb 1;85(3):313-24.
Bryostatin 1, like the phorbol esters, binds to and activates protein kinase C (PKC) but paradoxically antagonizes many but not all phorbol ester responses. Previously, we have compared patterns of biological response to bryostatin 1, phorbol ester, and the bryostatin 1 derivative Merle 23 in two human cancer cell lines, LNCaP and U937. Bryostatin 1 fails to induce a typical phorbol ester biological response in either cell line, whereas Merle 23 resembles phorbol ester in the U937 cells and bryostatin 1 in the LNCaP cells. Here, we have compared the pattern of their transcriptional response in both cell lines. We examined by qPCR the transcriptional response as a function of dose and time for a series of genes regulated by PKCs. In both cell lines bryostatin 1 differed primarily from phorbol ester in having a shorter duration of transcriptional modulation. This was not due to bryostatin 1 instability, since bryostatin 1 suppressed the phorbol ester response. In both cell lines Merle 23 induced a pattern of transcription largely like that of phorbol ester although with a modest reduction at later times in the LNCaP cells, suggesting that the difference in biological response of the two cell lines to Merle 23 lies downstream of this transcriptional regulation. For a series of bryostatins and analogs which ranged from bryostatin 1-like to phorbol ester-like in activity on the U937 cells, the duration of transcriptional response correlated with the pattern of biological activity, suggesting that this may provide a robust platform for structure activity analysis.
Comparison of effects of bryostatins 1 and 2 and 12-O-tetradecanoylphorbol-13-acetate on protein kinase C activity in A549 human lung carcinoma cells.[Pubmed:2720677]
Cancer Res. 1989 Jun 15;49(12):3242-5.
Activators of protein kinase C (PKC), such as 12-O-tetradecanoylphorbol-13-acetate (TPA) and bryostatins 1 and 2, inhibit the growth of A549 cells. At high concentrations the bryostatins do not affect cell growth. Here the hypothesis has been tested that modulation of A549 cell growth is the consequence of agent-induced changes in location or extent of cellular PKC activity. PKC activity was measured after semi-purification with nondenaturing polyacrylamide gel electrophoresis in the cytosol and the particulate fraction of A549 cells. When cells were exposed to TPA or mezerein, PKC activity underwent rapid and concentration-dependent translocation from the cytosol to the membrane. TPA at 0.1 microM or mezerein at 1 microM caused almost complete translocation within 30 min. Incubation with bryostatins 1 or 2 also led to enzyme translocation, which was, however, much weaker than that observed with the tumor promoters. Neither 4 alpha-phorboldidecanoate nor the synthetic diacylglycerols 1,2-sn-dioctanoylglycerol or 1-oleoyl-2-acetyl-sn-glycerol mimicked TPA in this way. Exposure of cells to TPA or the bryostatins for longer than 30 min caused the gradual disappearance of total cellular PKC activity. PKC downregulation was concentration dependent and complete after 24 h. A549 cells which had acquired temporary resistance toward the growth-arresting potential of TPA were completely devoid of any measurable PKC activity. The bryostatins were potent inhibitors of the binding of [3H]phorbol-12,13-dibutyrate to its receptors in intact cells, and the inhibition was dependent on bryostatin concentration. The results support the contention that PKC is involved in the mediation of growth inhibition caused by TPA or the bryostatins. However, the relationship between growth arrest and PKC translocation or downregulation seems to be a complex one.
Differential effects of bryostatins and phorbol esters on arachidonic acid metabolite release and epidermal growth factor binding in C3H 10T1/2 cells.[Pubmed:3132318]
Cancer Res. 1988 Jul 1;48(13):3702-8.
The bryostatins, a group of macrocyclic lactones isolated on the basis of their antineoplastic activity, protein kinase C in vitro and block phorbol ester binding to this enzyme. In some cellular systems, bryostatins mimic phorbol ester action. In other systems, however, the bryostatins display only marginal agonistic action and, instead, inhibit phorbol ester-induced responses. At least in primary mouse epidermal cells, a transient duration of action of bryostatin 1 could rationalize these differences. To determine whether this model of transient activation could explain the dual actions of bryostatin 1 in other cell systems, we have examined the effects of bryostatin 1 on short-term responses in C3H 10T1/2 mouse fibroblasts. Even at very short exposures (30 min), bryostatin 1 blocked phorbol ester-induced arachidonic acid metabolite release and induced only minimal release when assayed alone. In contrast, epidermal growth factor binding was markedly and rapidly decreased in bryostatin 1-treated C3H 10T1/2 cells, and this decrease showed only limited reversal 16 h after initial exposure. Bryostatins 2, 3, 4, 10, and several of their derivatives caused variable arachidonic acid metabolite release (10 to 60% of phorbol ester control) and correspondingly variable inhibition of phorbol ester action. Our findings on arachidonic acid metabolite release argue against transient activation of the protein kinase C pathway as the sole explanation of bryostatin 1 action. They indicate, moreover, differences in the structure-activity relations of the bryostatins for the phorbol ester-mimetic and phorbol ester-inhibitory actions.


