BombesinInvolved in gastrointestinal tract function CAS# 31362-50-2 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 31362-50-2 | SDF | Download SDF |
| PubChem ID | 16133891 | Appearance | Powder |
| Formula | C71H110N24O18S | M.Wt | 1619.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 1.43 mg/mL (0.88 mM; Need ultrasonic) | ||
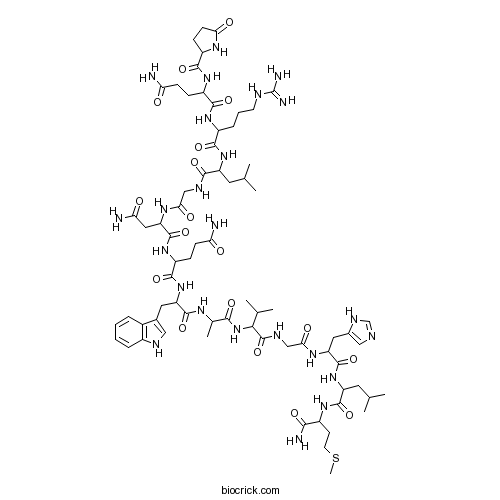
| Sequence | XQRLGNQWAVGHLM (Modifications: X-1 = Glp, Met-13 = C-terminal amide) | ||
| Chemical Name | N-[1-[[1-[[2-[[4-amino-1-[[5-amino-1-[[1-[[1-[[1-[[2-[[1-[[1-[(1-amino-4-methylsulfanyl-1-oxobutan-2-yl)amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1,4-dioxobutan-2-yl]amino]-2-oxoethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]-2-[(5-oxopyrrolidine-2-carbonyl)amino]pentanediamide | ||
| SMILES | CC(C)CC(C(=O)NCC(=O)NC(CC(=O)N)C(=O)NC(CCC(=O)N)C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)NC(C)C(=O)NC(C(C)C)C(=O)NCC(=O)NC(CC3=CNC=N3)C(=O)NC(CC(C)C)C(=O)NC(CCSC)C(=O)N)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCC(=O)N)NC(=O)C4CCC(=O)N4 | ||
| Standard InChIKey | QXZBMSIDSOZZHK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Neuropeptide with many biological effects including hormone release, stimulation of pancreatic enzyme secretion, inhibition of gastric emptying and modulation of gastric acid secretion. |

Bombesin Dilution Calculator

Bombesin Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bombesin is a tetradecapeptide originally isolated from frog skin; plays an important role in the release of gastrin and the activation of G-protein receptors. Sequence: {Glp}-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2.
In Vitro:Bombesin is a tetradecapeptide with a COOH terminus ending in Gly-His-Leu-Met-NH2 and subsequently is shown to closely resemble two mammalian bombesin -related peptides, gastrin-releasing peptide (GRP) and neuromedin B (NMB)[1]. Bombesin is found to have stimulatory effects upon gastric and pancreatic secretions, release of gastrointestinal hormones, gallbladder contraction and bronchoconstriction. It is present in amphibian gastric endocrine cells, avian proventriculus endocrine cells and avian brain. In mammals it is present mainly in nerve cells and fibers. The only mammalian endocrine cell shown to date to have bombesin is the P-cell in fetal lung. Bombesin is also found in mammalian brain, with its highest concentration in the hypothalamus[2]. Bombesin is shown to be a potent mitogen for Swiss 3T3 cells. In the presence of a low concentration (3.5%) of serum, bombesin stimulates 3T3 cell proliferation. In serum-free medium, bombesin induces DNA synthesis in the absence of any other added growth factor (IC50=1 nM)[3].
References:
[1]. Gonzalez N, et al. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008 Feb;15(1):58-64.
[2]. Chejfec G, et al. Bombesin in human neuroendocrine (NE) neoplasms. Peptides. 1985;6 Suppl 3:107-12.
- INH1
Catalog No.:BCC6040
CAS No.:313553-47-8
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- VU 590 dihydrochloride
Catalog No.:BCC7803
CAS No.:313505-85-0
- Reversan
Catalog No.:BCC7764
CAS No.:313397-13-6
- Regadenoson
Catalog No.:BCC6438
CAS No.:313348-27-5
- ICA 121431
Catalog No.:BCC6358
CAS No.:313254-51-2
- Aristolochic acid A
Catalog No.:BCN6262
CAS No.:313-67-7
- Estradiol Cypionate
Catalog No.:BCC4477
CAS No.:313-06-4
- Arjunic acid
Catalog No.:BCN5229
CAS No.:31298-06-3
- LDN-27219
Catalog No.:BCC6236
CAS No.:312946-37-5
- TCS JNK 5a
Catalog No.:BCC5148
CAS No.:312917-14-9
- Raucaffricine
Catalog No.:BCN4653
CAS No.:31282-07-2
- PD 118057
Catalog No.:BCC7499
CAS No.:313674-97-4
- 13-Oxo-9,11-octadecadienoic acid
Catalog No.:BCC8437
CAS No.:31385-09-8
- [Des-octanoyl]-Ghrelin (human)
Catalog No.:BCC7304
CAS No.:313951-59-6
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- o-3M3FBS
Catalog No.:BCC7210
CAS No.:313981-55-4
- PU 02
Catalog No.:BCC6265
CAS No.:313984-77-9
- [cPP1-7,NPY19-23,Ala31,Aib32,Gln34] - hPancreatic Polypeptide
Catalog No.:BCC5750
CAS No.:313988-89-5
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
A Selective Bombesin Receptor Subtype 3 Agonist Promotes Weight Loss in Male Diet-Induced-Obese Rats With Circadian Rhythm Change.[Pubmed:28324017]
Endocrinology. 2017 May 1;158(5):1298-1313.
Bombesin receptor subtype 3 (BRS-3) is an orphan G protein-coupled receptor. Based on the obese phenotype of male BRS-3-deficient mice, BRS-3 has been considered an attractive target for obesity treatment. Here, we developed a selective BRS-3 agonist (compound-A) and evaluated its antiobesity effects. Compound-A showed anorectic effects and enhanced energy expenditure in diet-induced-obese (DIO)-F344 rats. Moreover, repeated oral administration of compound-A for 7 days resulted in a significant body weight reduction in DIO-F344 rats. We also evaluated compound-A for cardiovascular side effects using telemeterized Sprague-Dawley (SD) rats. Oral administration of compound-A resulted in transient blood pressure increases in SD rats. To investigate the underlying mechanisms of BRS-3 agonist effects, we focused on the suprachiasmatic nucleus (SCN), the main control center of circadian rhythms in the hypothalamus, also regulating sympathetic nervous system. Compound-A significantly increased the messenger RNA expression of Brs-3, c-fos, and circadian rhythm genes in SCN of DIO-F344 rats. Because SCN also controls the hypothalamic-pituitary-adrenal (HPA) axis, we evaluated the relationship between BRS-3 and the HPA axis. Oral administration of compound-A caused a significant increase of plasma corticosterone levels in DIO-F344 rats. On this basis, energy expenditure enhancement by compound-A may be due to a circadian rhythm change in central and peripheral tissues, enhancement of peripheral lipid metabolism, and stimulation of the sympathetic nervous system. Furthermore, the blood pressure increase by compound-A could be associated with sympathetic nervous system stimulation via SCN and elevation of plasma corticosterone levels through activation of the HPA axis.
Bombesin Antagonist-Based Radiotherapy of Prostate Cancer Combined with WST-11 Vascular Targeted Photodynamic Therapy.[Pubmed:28108545]
Clin Cancer Res. 2017 Jul 1;23(13):3343-3351.
Purpose: DOTA-AR, a Bombesin-antagonist peptide, has potential clinical application for targeted imaging and therapy in gastrin-releasing peptide receptor (GRPr)-positive malignancies when conjugated with a radioisotope such as (90)Y. This therapeutic potential is limited by the fast washout of the conjugates from the target tumors. WST-11 (Weizmann STeba-11 drug; a negatively charged water-soluble palladium-bacteriochlorophyll derivative, Tookad Soluble) vascular targeted photodynamic therapy (VTP) is a local ablation approach recently approved for use in early-stage prostate cancer. It generates reactive oxygen/nitrogen species within tumor blood vessels, resulting in their instantaneous destruction followed by rapid tumor necrosis. We hypothesize that the instantaneous arrest of tumor vasculature may provide a means to trap radiopharmaceuticals within the tumor, thereby improving the efficacy of targeted radiotherapy.Experimental Design: GRPr-positive prostate cancer xenografts (PC-3 and VCaP) were treated with (90)Y-DOTA-AR with or without VTP. The uptake of radioisotopes was monitored by Cherenkov luminescence imaging (CLI). The therapeutic efficacy of the combined VTP and (90)Y-DOTA-AR in PC-3 xenografts was assessed.Results: CLI of (90)Y-DOTA-AR demonstrated longer retention of radiotracer within the VTP-treated PC-3 xenografts compared with the non-VTP-treated ones (P < 0.05) at all time points (24-144 hours) after (90)Y-DOTA-AR injection. A similar pattern of retention was observed in VCaP xenografts. When (90)Y-DOTA-AR administration was combined with VTP, tumor growth delay was significantly longer than for the control or the monotherapy groups.Conclusions: Tumor vascular arrest by VTP improves (90)Y-DOTA-AR retention in the tumor microenvironment thereby enhancing therapeutic efficacy. Clin Cancer Res; 23(13); 3343-51. (c)2017 AACR.
Synthesis and exploration of novel radiolabeled bombesin peptides for targeting receptor positive tumor.[Pubmed:28088445]
Peptides. 2017 Mar;89:17-34.
Increasing evidence of peptide receptor overexpression in various cancer cells, warrant the development of receptor specific radiolabeled peptides for molecular imaging and therapy in nuclear medicine. Gastrin-releasing-peptide (GRP) receptor, are overexpressed in a variety of human cancer cells. The present study report the synthesis and biological evaluation of new Bombesin (BBN) analogs, HYNIC-Asp-[Phe(13)]BBN(7-13)-NH-CH2-CH2-CH3:BA1, HYNIC-Pro-[Tyr(13)Met(14)]BBN(7-14)NH2:BA2 as prospective tumor imaging agent with compare to BBN(7-14)NH2:BS as standard. The pharmacophores were radiolabeled in high yields with (99m)Tc, characterized for their stability in serum and saline, cysteine/histidine and were found to be substantially stable. Internalization/externalization and receptor binding studies were assessed using MDA-MB-231 cells and showed high receptor binding-affinity and favourable internalization. Fluorescence studies revealed that BA1 changed the morphology of the cells and could localize in the nucleus more effectively than BA2/BS. Cell-viability studies displayed substantial antagonistic and nuclear-internalization effect of BA1. BA1 also exhibited antiproliferative effect on MDA-MB-231 cell by inducing apoptosis. In vivo behaviour of the radiopeptides was evaluated in GRP receptor positive tumor bearing mice. The (99m)Tc-BA1/(99m)Tc-BA2 demonstrated rapid blood/urinary clearance through the renal pathway and comparatively more significant tumor uptake image and favourable tumor-to-non-target ratios provided by (99m)Tc-BA1. The specificity of the in vivo uptake was confirmed by co-injection with BS. Moreover, (99m)Tc-BA1 provided a much clearer tumor image in scintigraphic studies than others. Thus the combination of favourable in vitro and in vivo properties renders BA1 as more potential antagonist Bombesin-peptide for targeting GRP-receptor positive tumor. These properties are encouraging to carry out further experiments for non-invasive receptor targeting potential diagnostinc and therapeutic agent for tumors.
Imaging of Prostate Cancer Using Gallium-68-Labeled Bombesin.[Pubmed:28267450]
PET Clin. 2017 Apr;12(2):159-171.
Nuclear medicine can play an important role in evaluating prostate cancer combining anatomical and functional information with hybrid techniques. Various PET radiopharmaceuticals have been used for targeting specific biological markers in prostate cancer. Research is ideally oriented towards the development of radiopharmaceuticals targeting antigens overexpressed in prostate cancer, as opposed to normal prostate tissue. In this regard, gastrin-releasing peptide receptors (GRPR) are excellent candidates. Bombesin analogues targeting the GRPR have been investigated. Gallium-68 ((68)Ga) is an interesting PET radioisotope due to several advantages, such as availability, ease of radiochemistry, half-life, and costs. The focus of this review is on (68)Ga-labeled Bombesin analogues in prostate cancer.
Two distinct receptor subtypes for mammalian bombesin-like peptides.[Pubmed:1726343]
Trends Neurosci. 1991 Dec;14(12):524-8.
The mammalian Bombesin-like peptides, gastrin-releasing peptide (GRP) and neuromedin B (NMB), are structurally related neuropeptides that elicit a wide spectrum of biological activities including regulation of smooth muscle contraction, stimulation of secretion, modulation of neural activity, and growth regulation. Earlier studies have shown that GRP and NMB are expressed in different regions of both the CNS and peripheral organs. Recent ligand-binding and molecular-cloning studies have revealed two pharmacologically distinct G-protein-coupled receptor subtypes for mammalian Bombesin-like peptides that have different relative affinities for GRP, NMB and Bombesin receptor antagonists. Similar to the peptide ligands, the two receptor subtypes are expressed in a distinct but overlapping set of CNS regions, some of which have been identified in functional studies as sites where Bombesin peptides elicit defined biological responses. Delineation of these peptide ligands and receptor subtypes will be important in future studies that explore the molecular basis for the heterogeneous nature of the responses to Bombesin observed in mammalian systems.
Bombesin: potential integrative peptide for feeding and satiety.[Pubmed:2199952]
Peptides. 1990 May-Jun;11(3):595-607.
The neuropeptide Bombesin (BBS) is examined with regard to possible designation as an integrative peptide. The term integrative peptide has been proposed to distinguish a subset of regulatory peptides. These peptides, distributed in the body and the brain, may function as hormones and neurotransmitters to integrate physiological and psychological functions. It is suggested that BBS may function as a peripheral and central satiety-inducing agent. The specific topics with regard to BBS include: feeding, satiety, and aversion; peripheral and central effects; learning, memory, and reward; route of injection; taste modulation; gastrointestinal activity; neurotransmitter status; mechanism and neuroanatomical site of action; and neural and humoral transmission.


