BestatinAminopeptidase inhibitor CAS# 58970-76-6 |
- Bestatin trifluoroacetate
Catalog No.:BCC3909
CAS No.:223763-80-2
- Fumagillin
Catalog No.:BCC2347
CAS No.:23110-15-8
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Bestatin hydrochloride
Catalog No.:BCC3908
CAS No.:65391-42-6
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure
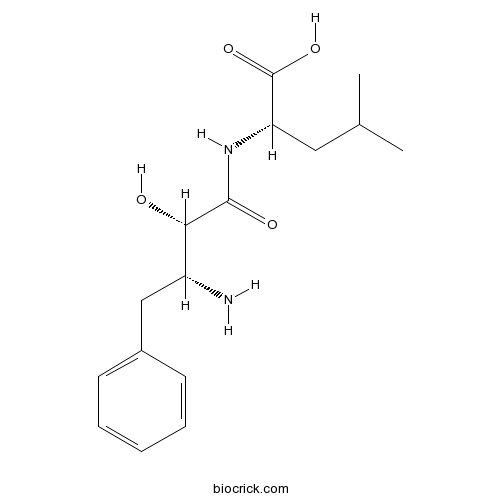
3D structure
| Cas No. | 58970-76-6 | SDF | Download SDF |
| PubChem ID | 72172 | Appearance | Powder |
| Formula | C16H24N2O4 | M.Wt | 308.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ubenimex | ||
| Solubility | DMSO : 8.33 mg/mL (27.01 mM; Need ultrasonic) | ||
| Chemical Name | (2S)-2-[[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic acid | ||
| SMILES | CC(C)CC(C(=O)O)NC(=O)C(C(CC1=CC=CC=C1)N)O | ||
| Standard InChIKey | VGGGPCQERPFHOB-RDBSUJKOSA-N | ||
| Standard InChI | InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aminopeptidase inhibitor (Ki = 0.001 - 90 mM); inhibits enkephalin metabolism and leukotriene A4 hydrolase. Inhibits tumor cell proliferation. |
| Cell experiment [1]: | |
| Cell lines | K562 and K562/ADR cells |
| Preparation method | The solubility of this compound in DMSO is <10 mm. general tips for obtaining a higher concentration: please warm the tube at 37 °c 10 minutes and> |
| Reacting condition | 24 h; 100 μM |
| Applications | To determine the interaction and the possible role of APN in MDR, RT–PCR was performed to detect the mRNA levels of APN and MDR1 in K562 and K562/ADR cells. After incubation with various concentration of bestatin for 24 h, the expression of APN mRNA was almost unchanged in K562 and K562/ADR cells. However, K562/ADR cells exhibited a significant lower level of APN mRNA than K562 cells. On the other hand, high dose of bestatin (100 μM) induced MDR1 upregulation by 49.4% and 18.0% in K562 and K562/ADR cells, respectively. The result confirmed that bestatin was a substrate of P-gp in mRNA level. |
| Animal experiment [1]: | |
| Animal models | Male Wistar rat |
| Dosage form | 4 mg/kg, dis-solved in normal saline; oral taken |
| Application | When bestatin and CsA were co-administered orally, the plasma concentrations of bestatin were increased significantly compared to that of control group. 1.97- and 1.92-fold increases were observed in Cmax (4.8±0.8 μg/ml vs. 2.4±0.6 μg/ml) and AUC (1.06±0.14 mg min/ml vs. 0.55±0.04 mg min/ml) of bestatin after combination with CsA, respectively. The results suggested concomitantly administered CsA increased the intestinal absorption of bestatin. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Huo X, Liu Q, Wang C, et al. Enhancement effect of P-gp inhibitors on the intestinal absorption and antiproliferative activity of bestatin[J]. European Journal of Pharmaceutical Sciences, 2013, 50(3): 420-428. | |

Bestatin Dilution Calculator

Bestatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2429 mL | 16.2143 mL | 32.4286 mL | 64.8572 mL | 81.0714 mL |
| 5 mM | 0.6486 mL | 3.2429 mL | 6.4857 mL | 12.9714 mL | 16.2143 mL |
| 10 mM | 0.3243 mL | 1.6214 mL | 3.2429 mL | 6.4857 mL | 8.1071 mL |
| 50 mM | 0.0649 mL | 0.3243 mL | 0.6486 mL | 1.2971 mL | 1.6214 mL |
| 100 mM | 0.0324 mL | 0.1621 mL | 0.3243 mL | 0.6486 mL | 0.8107 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Bestatin potentiated the humoral response to SRBC in mice increasing numbers of PFC AND 2-ME-resistant anti-SRBC antibodies and decreasing total anti-SRBC hemagglutinins; while it partially restored cyclophosphamide-induced immunosuppression in a dose-and-schedule-dependent manner.
Abstract
The intravenous co-administration of bestatin and MTX resulted in increased uptake of them in intestines and decreased uptake in kidney and hOAT1- or hOAT3-HEK 293 cells, which indicates that the interaction of these two drugs occurs through co-transport by P-gp in the intestinal mucosa and OATs in kidney.
Abstract
Reduced intestinal absorption and accumulation of bestatin was caused by P-gp-mediated efflux and could be restored by the addition of P-gp inhibitors (verapamil or Cyclosporin A) or P-gp substrates (doxorubicin) leading to decreased IC50 value and increased level of bestatin.
Abstract
Bestatin (Ubenimex) at high concentration inhibited proliferation of tumor cells (HT-1080) and induced apoptosis and cell arrest at G1 phase; while it at low concentration inhibited the aminopeptidase activity and invasion of tumor cells.
Abstract
OAT1 and OAT3 have been found to be involved in the renal excretion of bestatin, since reduced uptake of bestatin in hOAT1-HEK and hOAT3-HEK 293 cells were observed in the presence of OAT substrates including PAH, PCG and JBP485.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ubenimex(Bestatin) is a specific inhibitor of aminopeptidase B and leucine aminopeptidase. It did not show any inhibition of aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin or thermolysin. Bestatin at 100 pg/ml showed no antibacterial and no antifungal activities. It has low toxicity with no death after intraperitoneal injection of 300 mg/kg to mice1.
Bestatin isolated from the culture filtrate of Streptomyces olivoreticuli MD976-C72. The structure of bestatin was elucidated to be (2S, 3R)-3-amino-2-hydroxy-4- phenylbutanoyll-(S)-leucine3, 4. Bestatin itself was not hydrolyzed by either of the enzymes, when bestatin was incubated as substrate, L-leucine was not detected by thin-layer chromatography.
Unlike the case of orthophenanthroline, the inhibitory activity of bestatin on aminopeptidase B was not reversed by addition of zinc ion. Bestatin has a pair of adjacent amino and hydroxyl groups, which shows metal-complexing activity5-7. If the inhibitory activity of bestatin is attributable to five-membered chelate ring formation by a pair of adjacent amino and hydroxyl groups of bestatin and a metal ion of the enzyme, the isomers having erythro AHPA, which is difficult to form a chelate ring, are expected not to show inhibitory activity. However, the isomers having erythro-AHPA or (2S, 3S)-AHPA showed marked inhibitory activity. Bestatin and its active isomers are effective due to a mechanism other than a chelating action at the active center8.
References:
1. Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T, Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes, J Antibiot (Tokyo). 1976 Jan; 29(1):97-9.
2. Umezawa, H., Aoyagi, T., Suda, H., Hamada, M., And Takeuchi, T. 197615. Antibiotics 29, 97.
3. Suda, H., Takita, T., Aoyagi, T., And Umezawa, H. (1976) J. Antibiotics 29, 100.
4. Nakamura, H., Suda, H., Takita, T., Aoyagi, T., Umezawa, H., And Iitaka, Y. (1976) J. Antibiotics 29, 102.
5. Umezawa, S., Tsuchiya, T., And Tatsuta, K. (1966) Bull. Chem. Sot. Japan 39, 1235.
6. Barlow, C. B., And Gijthrie, R. D. (1967) J. Chem. Sot. (C) 1194.
7. Bukhari, S. T. K., Guthrie, R. D., Scott, A. I., And Wrixon, A. D. (1970) Tetrahedron 26, 3653.
8. Suda et al. Inhibition of aminopeptidase B and leucine aminopeptidase by bestatin and its stereoisomer, Archives of Biochemistry and Biophysics, 77, 196-200 (1976)
- Cyclizine 2HCl
Catalog No.:BCC4518
CAS No.:5897-18-7
- D-Phe-Ol
Catalog No.:BCC2580
CAS No.:58917-85-4
- Z-Glu-OtBu
Catalog No.:BCC2778
CAS No.:5891-45-2
- Cassythicine
Catalog No.:BCN5802
CAS No.:5890-28-8
- Laurolitsine
Catalog No.:BCN2634
CAS No.:5890-18-6
- Monomyristin
Catalog No.:BCN8388
CAS No.:589-68-4
- Nalmefene hydrochloride
Catalog No.:BCC7857
CAS No.:58895-64-0
- Arjungenin
Catalog No.:BCN8223
CAS No.:58880-25-4
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- Ophiopogonanone F
Catalog No.:BCN6409
CAS No.:588706-67-6
- Ophiopogonanone E
Catalog No.:BCN6625
CAS No.:588706-66-5
- 9-Oxonerolidol
Catalog No.:BCN5801
CAS No.:58865-88-6
- Xanthurenic acid
Catalog No.:BCC7866
CAS No.:59-00-7
- DL-alpha-Tocopherol
Catalog No.:BCN2200
CAS No.:59-02-9
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
- Ethopabate
Catalog No.:BCC8964
CAS No.:59-06-3
- 5-BrdU
Catalog No.:BCC5293
CAS No.:59-14-3
- D-Galactose
Catalog No.:BCN8528
CAS No.:59-23-4
- Folic acid
Catalog No.:BCN5375
CAS No.:59-30-3
- Mepyramine maleate
Catalog No.:BCC6740
CAS No.:59-33-6
- Sulfaquinoxaline
Catalog No.:BCC9158
CAS No.:59-40-5
- Thiamine chloride
Catalog No.:BCN8344
CAS No.:59-43-8
- Procaine
Catalog No.:BCC5210
CAS No.:59-46-1
- Oxindole
Catalog No.:BCN4050
CAS No.:59-48-3
Enhancement effect of resveratrol on the intestinal absorption of bestatin by regulating PEPT1, MDR1 and MRP2 in vivo and in vitro.[Pubmed:26394120]
Int J Pharm. 2015 Nov 10;495(1):588-598.
The purpose of present study was to assess the enhancing effect of resveratrol (Res) on the absorption of Bestatin and clarify the related molecular mechanism. Res facilitated Bestatin absorption by down-regulating both protein and gene levels of multidrug resistance 1 (Mdr1) and Multidrug resistance-associated protein 2 (Mrp2), and up-regulating oligopeptide transporter 1 (Pept1) protein and mRNA expression in rat intestine. In the same manner, Res increased penetration of Bestatin via significantly activating mRNA and protein expression of PEPT1 in Caco-2 cells. Conversely, mRNA and protein expression levels of MDR1, MRP2 and phosphorylation level of Insulin-like growth factor 1 receptor (IGF-1R) were inhibited by Res in Caco-2 cells. Moreover, Res also altered the phosphorylation of extracellular signal-regulated kinase (ERK) and protein kinase B (AKT). Res enhanced the intracellular concentration of Bestatin by down-regulating MDR1 and MRP2 expression through a mechanism that involves IGF-1R/AKT/ERK signaling pathway inhibition in Caco-2 cells. In conclusion, Res enhances Bestatin absorption by regulating PEPT1, MDR1 and MRP2 both in vivo and in vitro.
Protective effects of bestatin in the retina of streptozotocin-induced diabetic mice.[Pubmed:27344955]
Exp Eye Res. 2016 Aug;149:100-106.
CD13/APN (aminopeptidase N) was first identified as a selective angiogenic marker expressed in tumor vasculature and is considered a target for anti-cancer therapy. CD13 was also reported to express in non-diabetic, hypoxia-induced retinal neovascularization. Whether diabetes induces upregulation of CD13 expression in the retina is unknown. We hypothesize that at an early stage of non-proliferative diabetic retinopathy (NPDR) characterized by disruption of blood-retinal barrier (BRB) permeability is related to upregulated expression of CD13 because of its known role in extracellular matrix (ECM) degradation. The purpose of this study is to evaluate the role of CD13/APN and the therapeutic efficacy of a CD13/APN inhibitor in a mouse model of streptozotocin-induced NPDR. Hyperglycemic C57Bl/6 mice 26 weeks after streptozotocin (STZ) injection were intravitreally injected with a sustained release formulation of CD13/APN inhibitor Bestatin. At 15th day of post-Bestatin treatment, mouse retinas were evaluated for vascular permeability by Evans blue dye extravasation assay, fluorescent angiography of retinal vascular permeability and leukostasis. Retinal protein extracts were analyzed by Western blot to determine the effects of Bestatin treatment on the expression of CD13/APN related inflammatory mediators of ECM degradation and angiogenesis. Intravitreal Bestatin treatment significantly inhibited retinal vascular permeability and leukostasis. This treatment also significantly inhibited retinal expression of CD13, ECM degrading proteases (heparanase and MMP9 and angiogenic molecules (HIF-1alpha and VEGF). Intravitreal CD13 inhibition may relate to furthering our knowledge on the protective effect of Bestatin against diabetic retinal vasculature abnormalities through inhibition of retinal permeability, leukostasis, inflammatory molecules of ECM degradation and angiogenesis.
Degradation of HaloTag-fused nuclear proteins using bestatin-HaloTag ligand hybrid molecules.[Pubmed:26338696]
Org Biomol Chem. 2015 Oct 14;13(38):9746-50.
We have developed a protein knockdown technology using hybrid small molecules designed as conjugates of a ligand for the target protein and a ligand for ubiquitin ligase cellular inhibitor of apoptosis protein 1 (cIAP1). However, this technology has several limitations. Here, we report the development of a novel protein knockdown system to address these limitations. In this system, target proteins are fused with HaloTag to provide a common binding site for a degradation inducer. We designed and synthesized small molecules consisting of alkyl chloride as the HaloTag-binding degradation inducer, which binds to HaloTag, linked to BE04 (2), which binds to cIAP1. Using this system, we successfully knocked down HaloTag-fused cAMP responsive element binding protein 1 (HaloTag-CREB1) and HaloTag-fused c-jun (HaloTag-c-jun), which are ligand-unknown nuclear proteins, in living cells. HaloTag-binding degradation inducers can be synthesized easily, and are expected to be useful as biological tools for pan-degradation of HaloTag-fused proteins.
Resveratrol Increases Anti-Proliferative Activity of Bestatin Through Downregulating P-Glycoprotein Expression Via Inhibiting PI3K/Akt/mTOR Pathway in K562/ADR Cells.[Pubmed:26460589]
J Cell Biochem. 2016 May;117(5):1233-9.
Multidrug resistance (MDR) is a major obstacle in the clinical therapy of hematological malignancies. P-glycoprotein (P-gp) overexpression results in reduction of intracellular drug concentration with a consequence that the cytotoxicity of anti-tumor drugs is decreased, which leads to MDR in K562/ADR cells. In this study, we found that resveratrol enhanced the anti-proliferative activity of Bestatin in K562/ADR cells. Co-treatment with resveratrol, IC50 values of Bestatin in K562/ADR cells significantly decreased and activation of caspase-3 and caspase-8 increased, which indicated that resveratrol potentiated Bestatin-induced apoptosis. Resveratrol increased the intracellular concentration of Bestatin through inhibiting P-gp function and downregulating P-gp expression at mRNA and protein levels, which increased anti-proliferative activity of Bestatin in K562/ADR cells. Resveratrol decreased the phosphorylation of Akt and mTOR but did not affect the phosphorylations of JNK or ERK1/2. These results demonstrated that resveratrol could increase the anti-proliferative activity of Bestatin through downregulating P-gp expression via suppressing the PI3K/Akt/mTOR signaling pathway.
Effect of ubenimex (Bestatin) on the cell growth and phenotype of HL-60 and HL-60R cell lines: up-and down-regulation of CD13/aminopeptidase N.[Pubmed:11042531]
Leuk Lymphoma. 2000 May;37(5-6):663-7.
Ubenimex (Bestatin), a low-molecular-mass dipeptide, has been demonstrated to have anti-tumor activities and immunomodulating activities. We here report cell growth inhibition and phenotypic changes of HL-60 and HL-60R cell lines induced by Bestatin treatment. Bestatin (0.1 microg/ml) showed remarkable cell growth inhibition against HL-60 cells, whereas it was ineffective for HL 60R cells. Bestatin also showed growth inhibition in the concentration of 1 microg/ml against HL-60R cells which are resistant to differentiation induction by DMSO and TPA. In both cell types, the effect of growth inhibition by Bestatin treatment was dose dependent. We found a low level expression of CD13 and a low number of CD13 positive cells in HL-60R cells compared with that of HL-60. We also observed phenotypic changes of HL-60 and HL-60R cells following incubation with Bestatin (10 microg/ml) for 1 and 3 hrs, respectively. With HL-60 cells, the upregulation of CD13/aminopeptidase N was found after 1 hr, however, the downregulation was observed after 3 hrs incubation with Bestatin. On the other hand, the downregulation of CD15 and CD33 was observed after both one and 3 hrs incubation. Similarly, in HL-60R cells, the upregulation of CD13/aminopeptidase N was found temporarily (1hr), and then CD13 downregulation was observed after 3 hrs incubation with Bestatin. No notable change was observed for expression of other myeloid-related antigens, e.g. CD14 (My4, LeuM3), CD11b (OKM1), and CD34 (My10). On the basis of these observations of in vitro activity, we suggest that Bestatin may also be an effective anti-leukemic agent in vivo.


