BRAF inhibitorPotent B-raf inhibitor CAS# 918505-61-0 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
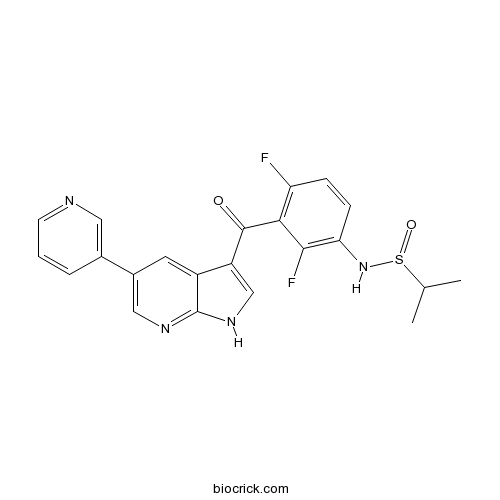
| Cas No. | 918505-61-0 | SDF | Download SDF |
| PubChem ID | 57345817 | Appearance | Powder |
| Formula | C22H18F2N4O3S | M.Wt | 456.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (109.54 mM; Need ultrasonic) | ||
| Chemical Name | N-[2,4-difluoro-3-(5-pyridin-3-yl-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)phenyl]propane-2-sulfinamide | ||
| SMILES | CC(C)S(=O)NC1=C(C(=C(C=C1)F)C(=O)C2=CNC3=NC=C(C=C23)C4=CN=CC=C4)F | ||
| Standard InChIKey | USQPXRQLUWVIAC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H18F2N4O2S/c1-12(2)31(30)28-18-6-5-17(23)19(20(18)24)21(29)16-11-27-22-15(16)8-14(10-26-22)13-4-3-7-25-9-13/h3-12,28H,1-2H3,(H,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

BRAF inhibitor Dilution Calculator

BRAF inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1907 mL | 10.9536 mL | 21.9072 mL | 43.8145 mL | 54.7681 mL |
| 5 mM | 0.4381 mL | 2.1907 mL | 4.3814 mL | 8.7629 mL | 10.9536 mL |
| 10 mM | 0.2191 mL | 1.0954 mL | 2.1907 mL | 4.3814 mL | 5.4768 mL |
| 50 mM | 0.0438 mL | 0.2191 mL | 0.4381 mL | 0.8763 mL | 1.0954 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.2191 mL | 0.4381 mL | 0.5477 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BRAF inhibitor is a potent BRAF inhibitor.
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- 5,7,4'-Trimethoxyafzelechin
Catalog No.:BCN7933
CAS No.:918428-88-3
- Cefdinir
Catalog No.:BCC3747
CAS No.:91832-40-5
- UCL 2077
Catalog No.:BCC7446
CAS No.:918311-87-2
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
- MK-2461
Catalog No.:BCC3816
CAS No.:917879-39-1
- CCMI
Catalog No.:BCC7788
CAS No.:917837-54-8
- PSB 0474
Catalog No.:BCC7459
CAS No.:917567-60-3
- Platycoside M3
Catalog No.:BCN3243
CAS No.:917482-69-0
- Platycoside M1
Catalog No.:BCN3238
CAS No.:917482-67-8
- Cyclo(Ile-Leu)
Catalog No.:BCN2434
CAS No.:91741-17-2
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
- TH-302
Catalog No.:BCC1998
CAS No.:918633-87-1
- GPi 688
Catalog No.:BCC6091
CAS No.:918902-32-6
- 19-[(beta-D-glucopyranosyl)oxy]-19-oxo-ent-labda-8(17),13-dien-16,15-olide
Catalog No.:BCN1308
CAS No.:919120-78-8
- 1-Methoxyindole-3-carboxylic acid
Catalog No.:BCN3946
CAS No.:91913-76-7
- Atrial natriuretic factor (1-28) (human, porcine)
Catalog No.:BCC5839
CAS No.:91917-63-4
- Ro 15-4513
Catalog No.:BCC7230
CAS No.:91917-65-6
- Rubrisandrin A
Catalog No.:BCN3248
CAS No.:919289-30-8
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
- Zatebradine hydrochloride
Catalog No.:BCC7286
CAS No.:91940-87-3
- Buergerinin B
Catalog No.:BCN4555
CAS No.:919769-83-8
- Saikosaponin H
Catalog No.:BCN7808
CAS No.:91990-63-5
Metastatic BRAF K601E-mutated melanoma reaches complete response to MEK inhibitor trametinib administered for over 36 months.[Pubmed:28344857]
Exp Hematol Oncol. 2017 Mar 21;6:6.
BACKGROUND: The BRAF K601E mutation occurs in 5% of patients with melanoma, and is the third most common type of BRAF mutation. However, treatment with BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors is only approved in patients with BRAF V600-positive melanoma, and patients with K601E-mutated melanoma do not have access to such drugs. CASE PRESENTATION: A female patient was diagnosed with high tumor burden metastatic melanoma harboring the BRAF K601E mutation. After chemotherapy failure, she underwent compassionate treatment with trametinib. Trametinib showed good activity and efficacy, with 48% shrinkage of a metastatic lymphadenopathy after 4 months' treatment. However, the patient reported treatment-related skin toxicity that required dosage reduction and a personalized intermittent trametinib dosing schedule. After over 36 months from the first trametinib administration, and resection of a metastatic lymphadenopathy, the patient experienced complete response. CONCLUSIONS: This case report shows that trametinib could be a valid therapeutic option in patients with metastatic melanoma harboring the rare BRAF K601E mutation.
Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAF(V600)-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial.[Pubmed:28268064]
Lancet Oncol. 2017 Apr;18(4):464-472.
BACKGROUND: Patients with BRAF(V600)-mutant melanoma benefit from treatment with the combination of BRAF and MEK inhibitors, but resistance and disease progression develops in most patients. Preclinical studies and case studies have indicated that acquired resistance to BRAF inhibition can be reversible. We aimed to assess the anti-tumour activity of rechallenge with BRAF plus MEK inhibition in a prospective clinical trial. METHODS: In this open-label, single arm, dual-centre, phase 2 academic study in Belgium, patients aged 18 years or older with BRAF(V600)-mutant melanoma who had previously progressed on BRAF inhibitors (with or without MEK inhibitors) and were off-treatment for at least 12 weeks, were treated with dabrafenib 150 mg orally twice per day plus trametinib 2 mg orally once per day. The primary endpoint was the proportion of patients with investigator-assessed overall response at any time (defined as complete response or partial response according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 confirmed on two occasions, at least 28 days after the first response was recorded). Analyses were done in the intention-to-treat population. The study is ongoing but no longer recruiting patients. This trial is registered with ClinicalTrials.gov, number NCT02296996. FINDINGS: Between April 5, 2014, and Feb 2, 2016, 25 patients were enrolled and initiated treatment in our study. A partial response was documented in eight (32%) of 25 patients (95% CI 15-54; six patients had progressed on previous treatment with dabrafenib plus trametinib and two patients had progressed on previous BRAF inhibitor monotherapy). Stable disease was noted in ten patients (40%; 95% CI 21-61). Rechallenge with dabrafenib plus trametinib was well tolerated. There were no unexpected or grade 4 or 5 treatment-related adverse events. Grade 3 adverse events occurred in two patients (8%; panniculitis [n=1] and pyrexia [n=1]). Serious adverse events which occurred on study were one patient with an Addison crisis triggered by grade 2 pyrexia symptoms that resolved after discontinuation of dabrafenib and trametinib. No patients died as a result of study treatment. INTERPRETATION: Rechallenge with dabrafenib plus trametinib showed anti-tumour activity in patients who had previously progressed on BRAF inhibitors and as such, rechallenge represents a potential new treatment option for these patients. FUNDING: Vlaamse Liga Tegen Kanker, Novartis.
Response of BRAF inhibitor-associated squamous cell lung carcinoma to drug withdrawal.[Pubmed:28252478]
Melanoma Res. 2017 Apr;27(2):159-163.
Vemurafenib and dabrafenib, two Food and Drug Administration-approved selective BRAF kinase inhibitors (BRAFi), have revolutionized the targeted therapy of cutaneous melanoma. Off-target effects of these drugs paradoxically activate the MAP kinase pathway in BRAF wild-type cells, leading to secondary malignancies. Although cutaneous squamous cell carcinomas are by far the most frequent, emergence of potentially life-threatening secondary tumors from other sites following prolonged therapy is a growing concern. Herein, we provide the first case report of squamous cell lung carcinoma apparently secondary to BRAFi developing in a metastatic melanoma patient on vemurafenib for 23 months. Subsequent BRAFi with dabrafenib for 5 months was accompanied by rapid lung cancer progression with 86% increase in diameter. Withdrawal of BRAFi as the only change in therapy resulted in partial response maintained for more than 8 months. Clinicians should be atuned to the risk of noncutaneous second malignancies induced by BRAFi, particularly in the setting of progression of an isolated lesion after prolonged therapy.
BRAF inhibitor discontinuation and rechallenge in advanced melanoma patients with a complete initial treatment response.[Pubmed:28240681]
Melanoma Res. 2017 Jun;27(3):281-287.
BRAF inhibitors (BRAFi), a targeted therapy, are used to treat metastatic late-stage melanomas harbouring the BRAF-V600 mutation (found in about 50% of melanomas). The targeted therapy is generally maintained until tumour progression or major toxicity occurs, although responses are often limited in time. It is unknown whether melanoma patients achieving a complete response with targeted therapy can safely discontinue treatment. We retrospectively observed the clinical course of patients with metastatic melanoma who discontinued BRAFi therapy after achieving a complete response and those with an incomplete response combined with surgical removal of the remaining tumours. We also evaluated the effectiveness of BRAFi in these patients after recurrence. In 11 patients, the best response was diagnosed after a median BRAFi treatment duration of 105 (29-341) days. The median follow-up after BRAFi initiation was 769 (435-1765) days. Recurrence was observed in all 11 patients (100%), median: 82 (27-322) days. Five patients achieved a complete response, with a median progression-free survival after cessation of 136.5 (34-322) days versus 82 (27-144) days for six patients with an incomplete response combined with surgical removal of remaining tumours. Baseline characteristics and time to best response and to discontinuation did not influence the rate of relapse. Subsequently, eight patients were rechallenged with a BRAFi. The median progression-free survival time after BRAFi rechallenge was 222.5 (15-425) days. The three remaining patients received treatments other than BRAFi. Our findings may be valuable with respect to ongoing clinical trials of combinations of targeted therapies and immunomodulatory antibodies.


