BI-847325dual inhibitor of MEK and Aurora kinases CAS# 1207293-36-4 |
- Ro3280
Catalog No.:BCC3962
CAS No.:1062243-51-9
- MLN0905
Catalog No.:BCC3961
CAS No.:1228960-69-7
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- Rigosertib sodium
Catalog No.:BCC4067
CAS No.:592542-60-4
- GSK461364
Catalog No.:BCC3788
CAS No.:929095-18-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1207293-36-4 | SDF | Download SDF |
| PubChem ID | 54577344 | Appearance | Powder |
| Formula | C29H28N4O2 | M.Wt | 464.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 36 mg/mL (77.49 mM) *"≥" means soluble, but saturation unknown. | ||
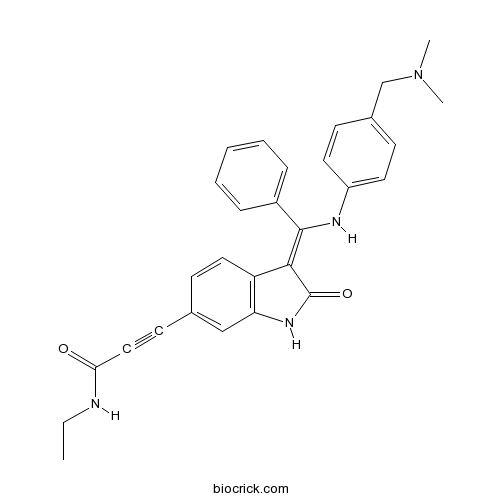
| Chemical Name | 3-[(3Z)-3-[[4-[(dimethylamino)methyl]anilino]-phenylmethylidene]-2-oxo-1H-indol-6-yl]-N-ethylprop-2-ynamide | ||
| SMILES | CCNC(=O)C#CC1=CC2=C(C=C1)C(=C(C3=CC=CC=C3)NC4=CC=C(C=C4)CN(C)C)C(=O)N2 | ||
| Standard InChIKey | FLBNLJLONKAPLR-DQSJHHFOSA-N | ||
| Standard InChI | InChI=1S/C29H28N4O2/c1-4-30-26(34)17-13-20-12-16-24-25(18-20)32-29(35)27(24)28(22-8-6-5-7-9-22)31-23-14-10-21(11-15-23)19-33(2)3/h5-12,14-16,18,31H,4,19H2,1-3H3,(H,30,34)(H,32,35)/b28-27- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BI-847325 is an ATP competitive dual inhibitor of MEK and aurora kinases (AK) with IC50 values of 4 and 15 nM for human MEK2 and AK-C, respectively.In Vitro:BI 847325 inhibits the activity of X. laevis AK-B with an IC50 of 3 nM; the IC50 values for human AK-A and AK-C are 25 and 15 nM, respectively. BI 847325 also inhibits human MEK1 and MEK2 with respective IC50 values of 25 and 4 nM. BI 847325 at 1,000 nM inhibits 6 enzymes by more than 50% (LCK, MAP3K8, FGFR1, AMPK, CAMK1D and TBK1) and the IC50 values are below 100 nM only for LCK (5 nM) and MAP3K8 (93 nM). Proliferation is inhibited in A375 and Calu-6 cell lines with GI50 values of 7.5 nM and 60 nM, respectively[1].In Vivo:Daily oral administration of BI 847325 at 10 mg/kg shows efficacy in both BRAF- and KRAS-mutant xenograft models. BI 847325 administered once weekly at 70 mg/kg inhibits both MEK and AK in KRAS-mutant tumors[1]. References: | |||||

BI-847325 Dilution Calculator

BI-847325 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1526 mL | 10.7629 mL | 21.5257 mL | 43.0515 mL | 53.8144 mL |
| 5 mM | 0.4305 mL | 2.1526 mL | 4.3051 mL | 8.6103 mL | 10.7629 mL |
| 10 mM | 0.2153 mL | 1.0763 mL | 2.1526 mL | 4.3051 mL | 5.3814 mL |
| 50 mM | 0.0431 mL | 0.2153 mL | 0.4305 mL | 0.861 mL | 1.0763 mL |
| 100 mM | 0.0215 mL | 0.1076 mL | 0.2153 mL | 0.4305 mL | 0.5381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BI 847325 is a dual inhibitor of Ras-mitogen-activated protein kinase kinases (MEK) and Aurora kinases [1,2]. BI 847325 inhibits MEK1 and MEK2 with IC50 values of 25 and 4 nM, respectively. For inhibiting the activity of Aurora A, B and C, IC50 values are 3, 25 and 15 nM, respectively [1].
The RAS-dependent MAP kinase signaling pathway is important in the regulation of cell survival and proliferation. It is hard to design direct inhibitors of RAS proteins. MEK is a downstream kinase of MAP kinase [1]. MEK is a ERK kinase [3].
In cells treated with BI 847325, based on levels of phospho-histone H3 (pHH3) and phospho-ERK (pERK), EC50 values were determined. To KRAS-and-PI3Kα-mutant NCI-H460 cells, the EC50 value was 44 nM. To BRAF-mutant A375 cells, the EC50 value was 37 nM. In a panel of 240 cell lines from diverse tissues with diverse genetic background, BI 847325 produced a potent inhibition of cell proliferation with a gm GI50 value of 28 nM. In a subset of cell lines, BI 847325 induced cell death. These in vitro potency correlated with mutations in BRAF or RAS, significantly [1].
In nude mouse xenograft models of cutaneous melanoma (A375, mutant BRAF) and NSCLC (Calu-6, mutant KRAS), BI 847325 at a daily oral dose of 10 mg/kg produced complete inhibition of tumor growth. In animals with A375 tumors, treatment with BI 847325 significantly reduced levels of both pHH3 and pERK in the tumors compared to controls [1].
References:
[1]. Sini P, Gürtler U, Zahn SK, et al. Pharmacological characterization of BI 847325, a dual inhibitor of MEK and Aurora kinases. Cancer Research, 2012, 72(8 Supplement): 1919-1919.
[2]. Hideshima T, Chauhan D, Richardson P, et al. NF-κB as a therapeutic target in multiple myeloma. Journal of Biological Chemistry, 2002, 277(19): 16639-16647.
[3]. Rommel C, Clarke BA, Zimmermann S, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science, 1999, 286(5445): 1738-1741..
- NVS-CRF38
Catalog No.:BCC8059
CAS No.:1207258-55-6
- 12alpha-Hydroxyevodol
Catalog No.:BCN6102
CAS No.:120722-04-5
- Sarcandrolide D
Catalog No.:BCN6621
CAS No.:1207185-03-2
- Scutebata G
Catalog No.:BCN6101
CAS No.:1207181-63-2
- Scutebata F
Catalog No.:BCN6100
CAS No.:1207181-62-1
- Scutebata E
Catalog No.:BCN6099
CAS No.:1207181-61-0
- Scutebata C
Catalog No.:BCN6098
CAS No.:1207181-59-6
- Scutebata B
Catalog No.:BCN6097
CAS No.:1207181-58-5
- Scutebata A
Catalog No.:BCN6096
CAS No.:1207181-57-4
- Psidial A
Catalog No.:BCN6095
CAS No.:1207181-35-8
- WAY 208466 dihydrochloride
Catalog No.:BCC7807
CAS No.:1207064-61-6
- (Z)-1-Methyl-2-(undec-6-enyl)quinolin-4(1H)-one
Catalog No.:BCN7063
CAS No.:120693-49-4
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- BMN 673
Catalog No.:BCC2205
CAS No.:1207456-01-6
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- LDV FITC
Catalog No.:BCC6229
CAS No.:1207610-07-8
- 3,2'-Epilarixinol
Catalog No.:BCN6496
CAS No.:1207671-28-0
- Huperzine A
Catalog No.:BCN1058
CAS No.:120786-18-7
- Gynosaponin I
Catalog No.:BCN4078
CAS No.:1207861-69-5
- Quassidine B
Catalog No.:BCN7022
CAS No.:1207862-37-0
- CaMKII-IN-1
Catalog No.:BCC5530
CAS No.:1208123-85-6
- VU 0365114
Catalog No.:BCC6164
CAS No.:1208222-39-2
Pharmacological Profile of BI 847325, an Orally Bioavailable, ATP-Competitive Inhibitor of MEK and Aurora Kinases.[Pubmed:27496137]
Mol Cancer Ther. 2016 Oct;15(10):2388-2398.
Although the MAPK pathway is frequently deregulated in cancer, inhibitors targeting RAF or MEK have so far shown clinical activity only in BRAF- and NRAS-mutant melanoma. Improvements in efficacy may be possible by combining inhibition of mitogenic signal transduction with inhibition of cell-cycle progression. We have studied the preclinical pharmacology of BI 847325, an ATP-competitive dual inhibitor of MEK and Aurora kinases. Potent inhibition of MEK1/2 and Aurora A/B kinases by BI 847325 was demonstrated in enzymatic and cellular assays. Equipotent effects were observed in BRAF-mutant cells, whereas in KRAS-mutant cells, MEK inhibition required higher concentrations than Aurora kinase inhibition. Daily oral administration of BI 847325 at 10 mg/kg showed efficacy in both BRAF- and KRAS-mutant xenograft models. Biomarker analysis suggested that this effect was primarily due to inhibition of MEK in BRAF-mutant models but of Aurora kinase in KRAS-mutant models. Inhibition of both MEK and Aurora kinase in KRAS-mutant tumors was observed when BI 847325 was administered once weekly at 70 mg/kg. Our studies indicate that BI 847325 is effective in in vitro and in vivo models of cancers with BRAF and KRAS mutation. These preclinical data are discussed in the light of the results of a recently completed clinical phase I trial assessing safety, tolerability, pharmacokinetics, and efficacy of BI 847325 in patients with cancer. Mol Cancer Ther; 15(10); 2388-98. (c)2016 AACR.
A phase I study of two dosing schedules of oral BI 847325 in patients with advanced solid tumors.[Pubmed:26650227]
Cancer Chemother Pharmacol. 2016 Jan;77(1):99-108.
PURPOSE: This study determined the safety, maximum tolerated dose (MTD), pharmacokinetics, and preliminary efficacy of BI 847325, an oral dual MEK and Aurora kinase inhibitor, in patients with refractory solid tumors. METHODS: This trial recruited patients with an advanced non-resectable and/or metastatic solid tumor following failure of conventional treatment (NCT01324830; 1287.1). BI 847325 was administered orally, once daily (starting at 6 mg in the first cohort) using two dosing schedules: Schedule A (2 weeks on, 1 week off) and Schedule B (three periods of 5 days on, 2 days off). The primary objective was to identify the MTD of BI 847325 for both dosing schedules. RESULTS: Sixty-nine patients (Schedule A, n = 47; Schedule B, n = 22) were treated. The MTD was 120 mg per day for Schedule A (cumulative dose of 1680 mg per 3-week cycle) and 150 mg per day for Schedule B (cumulative dose of 2250 mg per 3-week cycle). Reversible hematologic and gastrointestinal toxicities were the most common dose-limiting toxicities. One patient with esophageal cancer (receiving 160 mg BI 847325, Schedule A) experienced a partial response for 67 days, and 21 patients (n = 11 [23.4%], Schedule A; n = 10 [45.5%], Schedule B) had stable disease. Pharmacokinetic analyses showed at least bi-exponential disposition, with high inter-subject variability. There was no obvious relationship between markers of MEK or Aurora kinase inhibition and exposure to BI 847325 (exploratory analysis). CONCLUSIONS: This first-in-human trial suggests that BI 847325 has an acceptable safety profile. However, due to insufficient drug exposure at the MTD to achieve relevant MEK inhibition, a decision was taken to halt the development of BI 847325.
The Novel ATP-Competitive MEK/Aurora Kinase Inhibitor BI-847325 Overcomes Acquired BRAF Inhibitor Resistance through Suppression of Mcl-1 and MEK Expression.[Pubmed:25873592]
Mol Cancer Ther. 2015 Jun;14(6):1354-64.
Resistance to BRAF inhibitors is a major clinical problem. Here, we evaluate BI-847325, an ATP-competitive inhibitor of MEK and Aurora kinases, in treatment-naive and drug-resistant BRAF-mutant melanoma models. BI-847325 potently inhibited growth and survival of melanoma cell lines that were both BRAF inhibitor naive and resistant in 2D culture, 3D cell culture conditions, and in colony formation assays. Western blot studies showed BI-847325 to reduce expression of phospho-ERK and phospho-histone 3 in multiple models of vemurafenib resistance. Mechanistically, BI-847325 decreased the expression of MEK and Mcl-1 while increasing the expression of the proapoptotic protein BIM. Strong suppression of MEK expression was observed after 48 hours of treatment, with no recovery following >72 hours of washout. siRNA-mediated knockdown of Mcl-1 enhanced the effects of BI-847325, whereas Mcl-1 overexpression reversed this in both 2D cell culture and 3D spheroid melanoma models. In vivo, once weekly BI-847325 (70 mg/kg) led to durable regression of BRAF-inhibitor naive xenografts with no regrowth seen (>65 days of treatment). In contrast, treatment with the vemurafenib analog PLX4720 was associated with tumor relapse at >30 days. BI-847325 also suppressed the long-term growth of xenografts with acquired PLX4720 resistance. Analysis of tumor samples revealed BI-847325 to induce apoptosis associated with suppression of phospho-ERK, total MEK, phospho-Histone3, and Mcl-1 expression. Our studies indicate that BI-847325 is effective in overcoming BRAF inhibitor resistance and has long-term inhibitory effects upon BRAF-mutant melanoma in vivo, through a mechanism associated with the decreased expression of both MEK and Mcl-1.


