AtrasentanEndothelin antagonist receptor CAS# 173937-91-2 |
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Zibotentan (ZD4054)
Catalog No.:BCC2524
CAS No.:186497-07-4
- Atrasentan hydrochloride
Catalog No.:BCC1380
CAS No.:195733-43-8
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Macitentan
Catalog No.:BCC1142
CAS No.:441798-33-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 173937-91-2 | SDF | Download SDF |
| PubChem ID | 159594 | Appearance | Powder |
| Formula | C29H38N2O6 | M.Wt | 510.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
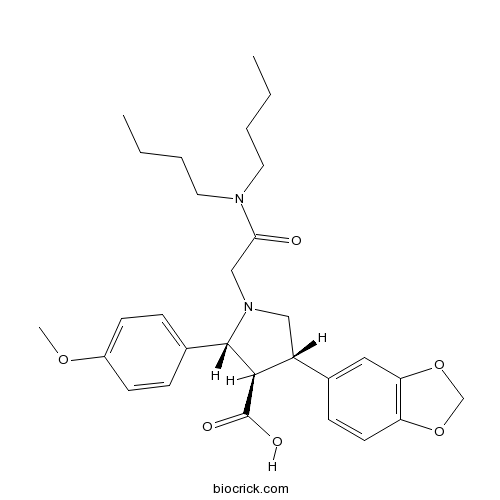
| Chemical Name | (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid | ||
| SMILES | CCCCN(CCCC)C(=O)CN1CC(C(C1C2=CC=C(C=C2)OC)C(=O)O)C3=CC4=C(C=C3)OCO4 | ||
| Standard InChIKey | MOTJMGVDPWRKOC-QPVYNBJUSA-N | ||
| Standard InChI | InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Atrasentan Dilution Calculator

Atrasentan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9584 mL | 9.792 mL | 19.584 mL | 39.1681 mL | 48.9601 mL |
| 5 mM | 0.3917 mL | 1.9584 mL | 3.9168 mL | 7.8336 mL | 9.792 mL |
| 10 mM | 0.1958 mL | 0.9792 mL | 1.9584 mL | 3.9168 mL | 4.896 mL |
| 50 mM | 0.0392 mL | 0.1958 mL | 0.3917 mL | 0.7834 mL | 0.9792 mL |
| 100 mM | 0.0196 mL | 0.0979 mL | 0.1958 mL | 0.3917 mL | 0.4896 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.0551 nM (for ET A receptor) [1] Atrasentan (A-147627) is an endothelin antagonist receptor being developed at Abbott Laboratories for the treatment of prostate cancer. in vitro: The combination of Atrasentan with Taxotere was more effective in the inhibition of cell viability and induction of apoptosis in LNCaP and C4-2b cells (androgen receptor positive) but not in PC-3 cells[2]. Atrasentan profoundly induced several CYPs and drug transporters (e.g. 12-fold induction of CYP3A4 at 50 μM). It was a moderate P-gp inhibitor (IC(50) in P388/dx cells = 15.1 ± 1.6 μM) and a weak BCRP inhibitor (IC(50) in MDCKII-BCRP cells = 59.8 ± 11 μM). BCRP or P-gp overexpressing cells were slightly more resistant towards antiproliferative effects of atrasentan [5]. in vivo: ABT-627 did reduce the accumulation of macrophages in both stains (36 to 53%) whereas it blocked by 76% the influx of eosinophils in Balb/c but not in C57Bl/6 mice [3]. Atrasentan was administered orally via drinking water at 3 mg kg-1 per day over 28 days. All diabetic mice developed similar hyperglycaemia (27-30 mmol l-1). Atrasentan treatment significantly improved left ventricular systolic and diastolic function in response to exogenous norepinephrine, but there were no differences between genotypes [4]. Clinical trial: Atrasentan and Zometa for Men With Prostate Cancer Metastatic to Bone . Phase2
- Gambogenic acid
Catalog No.:BCN3077
CAS No.:173932-75-7
- Corynoxine B
Catalog No.:BCN8454
CAS No.:17391-18-3
- Isocarapanaubine
Catalog No.:BCN1117
CAS No.:17391-09-2
- FR 171113
Catalog No.:BCC7734
CAS No.:173904-50-2
- Y-39983 dihydrochloride
Catalog No.:BCC4186
CAS No.:173897-44-4
- Swertiamarin
Catalog No.:BCN1116
CAS No.:17388-39-5
- H-Leu-OBzl.TosOH
Catalog No.:BCC2970
CAS No.:1738-77-8
- H-Gly-OBzl.TosOH
Catalog No.:BCC2948
CAS No.:1738-76-7
- H-Ser-OBzl.HCl
Catalog No.:BCC3030
CAS No.:1738-72-3
- H-Gly-OBzl.HCl
Catalog No.:BCC2949
CAS No.:1738-68-7
- Gambogin
Catalog No.:BCN3069
CAS No.:173792-67-1
- BGC 20-761
Catalog No.:BCC7650
CAS No.:17375-63-2
- Isogambogenin
Catalog No.:BCN3066
CAS No.:173938-23-3
- SYM 2206
Catalog No.:BCC6866
CAS No.:173952-44-8
- Tanshinone IIB
Catalog No.:BCN1118
CAS No.:17397-93-2
- 22-Hydroxy-3-oxo-12-ursen-30-oic acid
Catalog No.:BCN1526
CAS No.:173991-81-6
- 5-Acetyl-6-hydroxy-2-(1-hydroxy-1-methylethyl)benzofuran
Catalog No.:BCN7495
CAS No.:173992-05-7
- Nepicastat
Catalog No.:BCC1795
CAS No.:173997-05-2
- Dehydroabietic acid
Catalog No.:BCN1119
CAS No.:1740-19-8
- Bevirimat
Catalog No.:BCC5312
CAS No.:174022-42-5
- Tomatine
Catalog No.:BCN2966
CAS No.:17406-45-0
- 3-(4-Pyridyl)-D-Alanine.2HCl
Catalog No.:BCC2650
CAS No.:174096-41-4
- Aristolochic acid D
Catalog No.:BCN2902
CAS No.:17413-38-6
- 3-Chloro-4-hydroxypiperidin-2-one
Catalog No.:BCN3992
CAS No.:174204-83-2
Comparison of exposure response relationship of atrasentan between North American and Asian populations.[Pubmed:27981738]
Diabetes Obes Metab. 2017 Apr;19(4):545-552.
AIMS: The selective endothelin (ET) A receptor antagonist Atrasentan has been shown to lower albuminuria in North American and Asian patients with type 2 diabetes and nephropathy. As drug responses to many drugs may differ between North American and Asian populations, we assessed the influence of geographical region on the albuminuria and fluid retention response to Atrasentan. MATERIALS AND METHODS: Two 12-week double-blind randomised controlled trials were performed with Atrasentan 0.75 or 1.25 mg/d vs placebo in patients with type 2 diabetes and nephropathy. The efficacy endpoint was the percentage change in albuminuria. Bodyweight change, a proxy of fluid retention, was used as a safety endpoint. Pharmacodynamics were determined in Asians (N = 77) and North Americans (N = 134). Atrasentan plasma concentration was measured in 161 Atrasentan-treated patients. RESULTS: Mean albuminuria reduction in Asian, compared to North American, patients was, respectively, -34.4% vs -26.3% for 0.75 mg/d ( P = .44) and -48.0% vs -28.9% for 1.25 mg/d ( P = .035). Bodyweight gain did not differ between North American and Asian populations. Atrasentan plasma concentrations were higher in Asians compared to North Americans and correlated with albuminuria response (7.2% albuminuria reduction per doubling Atrasentan concentration; P = .024). Body surface area (beta = -1.09 per m(2) ; P < .001) and bilirubin, as a marker of hepatic organic anion transporter activity, (beta = 0.69 per mg/dL increment; P = .010) were independent determinants of Atrasentan plasma concentration; correction by body surface area and bilirubin left no significant difference in plasma concentration between Asian and North American populations. CONCLUSION: The higher exposure and albuminuria reduction of Atrasentan in Asian patients is not associated with more fluid retention, suggesting that Asian patients are less sensitive to Atrasentan-induced sodium retention.
The effects of atrasentan on urinary metabolites in patients with type 2 diabetes and nephropathy.[Pubmed:28019071]
Diabetes Obes Metab. 2017 May;19(5):749-753.
We assessed the effect of Atrasentan therapy on a pre-specified panel of 13 urinary metabolites known to reflect mitochondrial function in patients with diabetic kidney disease. This post-hoc analysis was performed using urine samples collected during the RADAR study which was a randomized, double-blind, placebo-controlled trial that tested the effects of Atrasentan on albuminuria reduction in patients with type 2 diabetes and nephropathy. At baseline, 4 of the 13 metabolites, quantified by gas-chromatography mass spectrometry, were below detectable levels, and 6 were reduced in patients with eGFR < 60 mL/min/1.73 m(2) . After 12 weeks of Atrasentan treatment in patients with eGFR < 60 mL/min/1.73 m(2) , a single-value index of the metabolites changed by -0.31 (95%CI -0.60 to -0.02; P = .035), -0.08 (-12 to 0.29; P = .43) and 0.01 (-0.21 to 0.19; P = .913) in placebo, Atrasentan 0.75 and 1.25 mg/d, respectively. The metabolite index difference compared to placebo was 0.13 (-0.17 to 0.43; P = .40) and 0.35 (0.05-0.65; P = .02) for Atrasentan 0.75 and 1.25 mg/d, respectively. These data corroborate previous findings of mitochondrial dysfunction in patients with type 2 diabetes, nephropathy and eGFR < 60 mL/min/1.73 m(2) , suggesting that Atrasentan may prevent the progression of mitochondrial dysfunction common to this specific patient population. Future studies of longer treatment duration with Atrasentan are indicated.
Atrasentan Reduces Albuminuria by Restoring the Glomerular Endothelial Glycocalyx Barrier in Diabetic Nephropathy.[Pubmed:27207530]
Diabetes. 2016 Aug;65(8):2429-39.
Atrasentan, a selective endothelin A receptor antagonist, has been shown to reduce albuminuria in type 2 diabetes. We previously showed that the structural integrity of a glomerular endothelial glycocalyx is required to prevent albuminuria. Therefore we tested the potential of Atrasentan to stabilize the endothelial glycocalyx in diabetic apolipoprotein E (apoE)-deficient mice in relation to its antialbuminuric effects. Treatment with Atrasentan (7.5 mg/kg/day) for 4 weeks reduced urinary albumin-to-creatinine ratios by 26.0 +/- 6.5% (P < 0.01) in apoE knockout (KO) mice with streptozotocin-induced diabetes consuming an atherogenic diet, without changes in gross glomerular morphology, systemic blood pressure, and blood glucose concentration. Endothelial cationic ferritin surface coverage, investigated using large-scale digital transmission electron microscopy, revealed that Atrasentan treatment increases glycocalyx coverage in diabetic apoE KO mice from 40.7 +/- 3.2% to 81.0 +/- 12.5% (P < 0.05). This restoration is accompanied by increased renal nitric oxide concentrations, reduced expression of glomerular heparanase, and a marked shift in the balance of M1 and M2 glomerular macrophages. In vitro experiments with endothelial cells exposed to laminar flow and cocultured with pericytes confirmed that Atrasentan reduced endothelial heparanase expression and increased glycocalyx thickness in the presence of a diabetic milieu. Together these data point toward a role for the restoration of endothelial function and tissue homeostasis through the antialbuminuric effects of Atrasentan, and they provide a mechanistic explanation for the clinical observations of reduced albuminuria with Atrasentan in diabetic nephropathy.


