Angiotensin 1/2 + A (2 - 8)Potent endogenous vasoconstrictor peptide - derivative of angiotensin II CAS# 51833-76-2 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 51833-76-2 | SDF | Download SDF |
| PubChem ID | 71464379 | Appearance | Powder |
| Formula | C49H71N13O10 | M.Wt | 1002.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
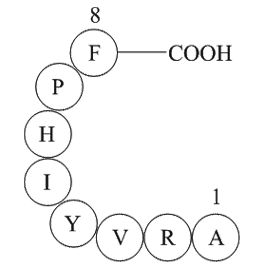
| Sequence | ARVYIHPF | ||
| Chemical Name | 2-[[1-[2-[[2-[[2-[[2-[[2-(2-aminopropanoylamino)-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-phenylpropanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CC1=CN=CN1)C(=O)N2CCCC2C(=O)NC(CC3=CC=CC=C3)C(=O)O)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(C(C)C)NC(=O)C(CCCN=C(N)N)NC(=O)C(C)N | ||
| Standard InChIKey | CHCOFDZSJYMQMX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C49H71N13O10/c1-6-28(4)40(46(69)58-36(24-32-25-53-26-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)39(27(2)3)60-42(65)34(56-41(64)29(5)50)14-10-20-54-49(51)52/h7-9,12-13,16-19,25-29,34-40,63H,6,10-11,14-15,20-24,50H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,51,52,54) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent endogenous vasoconstrictor peptide; derivative of angiotensin (Ang) II. Elicits pressor and renal vasoconstrictor effects in rodents via the AT1 receptor; inhibited by Candesartan but not by AT2 receptor ligands in vivo. Displays a similar affinity for AT1 and AT2 receptors as angiotensin II in vitro (Ki values are 1.6 and 2.3 nM at AT1 and AT2 receptors); also increases inositol phosphate accumulation with a similar potency to Ang II (EC50 = 6.7 nM). |

Angiotensin 1/2 + A (2 - 8) Dilution Calculator

Angiotensin 1/2 + A (2 - 8) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin 1/2 + A (2 - 8)
- UMI-77
Catalog No.:BCC5567
CAS No.:518303-20-3
- 2',4'-Dihydroxychalcone
Catalog No.:BCN5647
CAS No.:1776-30-3
- KX1-004
Catalog No.:BCC5440
CAS No.:518058-84-9
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Oxoepistephamiersine
Catalog No.:BCN5645
CAS No.:51804-68-3
- Nimesulide
Catalog No.:BCC4435
CAS No.:51803-78-2
- 3,3'-Di-O-methylellagic acid 4'-glucoside
Catalog No.:BCN1431
CAS No.:51803-68-0
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
- Cycleanine
Catalog No.:BCN8445
CAS No.:518-94-5
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- Emodin
Catalog No.:BCN5649
CAS No.:518-82-1
- Angiotensin (1-7)
Catalog No.:BCC1029
CAS No.:51833-78-4
- 3-MATIDA
Catalog No.:BCC7281
CAS No.:518357-51-2
- Allamandicin
Catalog No.:BCN4625
CAS No.:51838-83-6
- Licoricone
Catalog No.:BCN6818
CAS No.:51847-92-8
- 7-O-Methyleriodictyol
Catalog No.:BCN5648
CAS No.:51857-11-5
- Axillarin
Catalog No.:BCN8102
CAS No.:5188-73-8
- Pizotifen Malate
Catalog No.:BCC4825
CAS No.:5189-11-7
- Matrine
Catalog No.:BCN5650
CAS No.:519-02-8
- Benzoylecgonine
Catalog No.:BCN1909
CAS No.:519-09-5
- Ellipticine
Catalog No.:BCC7665
CAS No.:519-23-3
- Maclurin
Catalog No.:BCN5651
CAS No.:519-34-6
- Sulochrin
Catalog No.:BCN6959
CAS No.:519-57-3
Angiotensin-(1-7)-mediated Mas1 receptor/NF-kappaB-p65 signaling is involved in a cigarette smoke-induced chronic obstructive pulmonary disease mouse model.[Pubmed:28960804]
Environ Toxicol. 2018 Jan;33(1):5-15.
Angiotensin-(1-7) [Ang-(1-7)] has been shown to play a significant role in the pathogenesis of lung inflammation via Mas receptor; however, its effect in chronic obstructive pulmonary disease (COPD) remains unknown. To explore the effect of Ang-(1-7) on a cigarette smoke (CS) exposure-induced COPD model, 40 C57BL/6J mice were divided into four groups (n = 10) and exposed to air or CS for 8 weeks. After that, they were treated with saline or Ang-(1-7) at 0.3 mg/kg for 2 weeks by subcutaneous infusion using osmotic pump. The day following drug/vehicle challenge, lung function was examined and bronchoalveolar lavage (BAL) was performed. Chemokine (C-X-C motif) ligand 1, interleukin-6, and tumor necrosis factor-alpha protein levels in BAL fluid were determined using ELISA; the corresponding mRNA levels in lung tissues were measured using RT-PCR. Mas1 receptor, pIkappaBalpha, IkappaBalpha, nuclear NF-kappaB-p65 protein, pERK1/2, ERK2, pp38, and p38 proteins expression in lung tissues were examined by immunohistochemical staining and western blotting. Ang-(1-7) challenge had no effect on the decreased lung function and emphysema induced by CS exposure. However, Ang-(1-7) treatment blocked CS exposure-induced lung inflammatory responses and lung fibrosis, as determined by Masson's Trichrome staining. Exposure to CS for 8 weeks caused irreversible loss of lung function and emphysema, which could not be reversed by Ang-(1-7) treatment. Thus, the beneficial effect of Ang-(1-7) may be confined to pulmonary inflammation and fibrosis.
Activation pattern of ACE2/Ang-(1-7) and ACE/Ang II pathway in course of heart failure assessed by multiparametric MRI in vivo in Tgalphaq*44 mice.[Pubmed:28970203]
J Appl Physiol (1985). 2018 Jan 1;124(1):52-65.
Here, we analyzed systemic (plasma) and local (heart/aorta) changes in ACE/ACE-2 balance in Tgalphaq*44 mice in course of heart failure (HF). Tgalphaq*44 mice with cardiomyocyte-specific Galphaq overexpression and late onset of HF were analyzed at different age for angiotensin pattern in plasma, heart, and aorta using liquid chromatography/mass spectrometry, for progression of HF by in vivo magnetic resonance imaging under isoflurane anesthesia, and for physical activity by voluntary wheel running. Six-month-old Tgalphaq*44 mice displayed decreased ventricle radial strains and impaired left atrial function. At 8-10 mo, Tgalphaq*44 mice showed impaired systolic performance and reduced voluntary wheel running but exhibited preserved inotropic reserve. At 12 mo, Tgalphaq*44 mice demonstrated a severe impairment of basal cardiac performance and modestly compromised inotropic reserve with reduced voluntary wheel running. Angiotensin analysis in plasma revealed an increase in concentration of angiotensin-(1-7) in 6- to 10-mo-old Tgalphaq*44 mice. However, in 12- to 14-mo-old Tgalphaq*44 mice, increased angiotensin II was noted with a concomitant increase in Ang III, Ang IV, angiotensin A, and angiotensin-(1-10). The pattern of changes in the heart and aorta was also compatible with activation of ACE2, followed by activation of the ACE pathway. In conclusion, mice with cardiomyocyte Galphaq protein overexpression develop HF that is associated with activation of the systemic and the local ACE/Ang II pathway. However, it is counterbalanced by a prominent ACE2/Ang-(1-7) activation, possibly allowing to delay decompensation. NEW & NOTEWORTHY Changes in ACE/ACE-2 balance were analyzed based on measurements of a panel of nine angiotensins in plasma, heart, and aorta of Tgalphaq*44 mice in relation to progression of heart failure (HF) characterized by multiparametric MRI and exercise performance. The early stage of HF was associated with upregulation of the ACE2/angiotensin-(1-7) pathway, whereas the end-stage HF was associated with downregulation of ACE2/angiotensin-(1-7) and upregulation of the ACE/Ang II pathway. ACE/ACE-2 balance seems to determine the decompensation of HF in this model.
A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome.[Pubmed:28877748]
Crit Care. 2017 Sep 7;21(1):234.
BACKGROUND: Renin-angiotensin system (RAS) signaling and angiotensin-converting enzyme 2 (ACE2) have been implicated in the pathogenesis of acute respiratory distress syndrome (ARDS). We postulated that repleting ACE2 using GSK2586881, a recombinant form of human angiotensin-converting enzyme 2 (rhACE2), could attenuate acute lung injury. METHODS: We conducted a two-part phase II trial comprising an open-label intrapatient dose escalation and a randomized, double-blind, placebo-controlled phase in ten intensive care units in North America. Patients were between the ages of 18 and 80 years, had an American-European Consensus Criteria consensus diagnosis of ARDS, and had been mechanically ventilated for less than 72 h. In part A, open-label GSK2586881 was administered at doses from 0.1 mg/kg to 0.8 mg/kg to assess safety, pharmacokinetics, and pharmacodynamics. Following review of data from part A, a randomized, double-blind, placebo-controlled investigation of twice-daily doses of GSK2586881 (0.4 mg/kg) for 3 days was conducted (part B). Biomarkers, physiological assessments, and clinical endpoints were collected over the dosing period and during follow-up. RESULTS: Dose escalation in part A was well-tolerated without clinically significant hemodynamic changes. Part B was terminated after 39 of the planned 60 patients following a planned futility analysis. Angiotensin II levels decreased rapidly following infusion of GSK2586881, whereas angiotensin-(1-7) and angiotensin-(1-5) levels increased and remained elevated for 48 h. Surfactant protein D concentrations were increased, whereas there was a trend for a decrease in interleukin-6 concentrations in rhACE2-treated subjects compared with placebo. No significant differences were noted in ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, oxygenation index, or Sequential Organ Failure Assessment score. CONCLUSIONS: GSK2586881 was well-tolerated in patients with ARDS, and the rapid modulation of RAS peptides suggests target engagement, although the study was not powered to detect changes in acute physiology or clinical outcomes. TRIAL REGISTRATION: ClinicalTrials.gov, NCT01597635 . Registered on 26 January 2012.
Association of Angiotensin-Converting Enzyme Gene Polymorphisms and Nephropathy in Diabetic Patients at a Tertiary Care Centre in South India.[Pubmed:28890661]
Clin Med Insights Endocrinol Diabetes. 2017 Aug 29;10:1179551417726779.
BACKGROUND: Genetic polymorphisms of the angiotensin-renin pathway have been thought to influence the development of diabetic nephropathy. However, there are conflicting results regarding this association in previous studies on populations with varying ethnicity. AIMS: Primary aim was to compare the frequency of distribution of angiotensin-converting enzyme (ACE) gene (insertion/deletion [I/D]) polymorphism in Tamilian Indian type 2 diabetic individuals with and without microalbuminuria. Secondary objective was to compare the frequency of distribution of the 3 genotypes in diabetic patients with urinary albumin/creatinine ratio (ACR) < 30 mg/dL, urinary ACR = 30 to 300 mg/dL, and urinary ACR > 300 mg/dL. METHODS: A total of 179 consecutive diabetic individuals between 40 and 70 years, from Puducherry and Tamilnadu of Dravidian descent participated in the study conducted from 2012 to 2014. Inclusion criteria were as follows: age >/= 40 years and duration of type 2 diabetes mellitus for >/=5 years. Patients were divided into 2 groups based on ACR values. Group 1 consisted of 50 individuals with urinary ACR < 30 mg/g of creatinine, and group 2 consisted of 129 individuals with urinary ACR > 30 mg/g. Angiotensin I-converting enzyme (ACE) gene polymorphism was determined by allele-specific polymerase chain reaction method using a primer pair flanking the polymorphic region of its intron 16. Furthermore, group 2 patients were subdivided into those with urinary ACR = 30 to 300 mg/g of creatinine and those with urinary ACR > 300 mg/g of creatinine, and distribution of ACE gene polymorphism was compared in the three groups. STATISTICS: Statistical analysis was done using SPSS version 17.0. Independent Student t test was used to compare mean values between the 2 groups. Odds ratio was calculated for testing association between ACE gene (I/D) polymorphism and presence of microalbuminuria. P < .05 was considered significant. Comparison of ACE genotypes among 3 groups of patients (ACR < 30 mg/g, ACR = 30-300 mg/g, and ACR > 300 mg/g) was done using 1-way analysis of variance with Bonferroni multiple comparison test as post hoc analysis. CONCLUSIONS: Heterozygous I/D genotype was more frequent in the study population (45.8%) than the other genotypes. There was no difference in the genotype distribution in patients with varying levels of albuminuria.
Early Supplementation of d-Cysteine or l-Cysteine Prevents Hypertension and Kidney Damage in Spontaneously Hypertensive Rats Exposed to High-Salt Intake.[Pubmed:28981205]
Mol Nutr Food Res. 2018 Jan;62(2).
SCOPE: We investigate whether early supplementation of precursors of hydrogen sulfide (H2 S), d- or l-cysteine can prevent hypertension and kidney damage in spontaneously hypertensive rats (SHR) treated with high-salt. METHODS AND RESULTS: We examine 12-week-old male SHRs from four groups: SHR, high salt SHR (SHRs received 1% NaCl in drinking water for 8 weeks), high salt SHR+d (SHRs received high salt and d-cysteine), and high salt SHR+l (SHRs received high salt and l-cysteine). d- or l-cysteine was supplemented at 8 mmol kg(-1) body weight/day between 4 and 6 weeks of ages. High salt intake exacerbate hypertension and kidney damage in SHRs, which is prevented by d- or l-cysteine supplementation. d- or l-Cysteine supplementation reduce the degree of high salt-induced oxidative stress damage. Renal 3-mercaptopyruvate sulphurtransferase (3MST) protein levels and activity are reduced by d- or l-cysteine supplementation. Additionally, d- or l-Cysteine supplementation reduce renal angiotensin I and angiotensin II concentrations, decrease mRNA expression of Ren, and increase protein levels of type 2 angiotensin II receptor. CONCLUSION: Early supplementation of d- or l-cysteine before hypertension becomes evident and may protect against hypertension and kidney damage in adult SHRs exposed to high salt consumption via regulation of oxidative stress, renin-angiotensin system, and H2 S-generating pathways.
Pressor and renal hemodynamic effects of the novel angiotensin A peptide are angiotensin II type 1A receptor dependent.[Pubmed:21464395]
Hypertension. 2011 May;57(5):956-64.
Recently, a new derivative of angiotensin (Ang) II, called "Ang A," has been discovered to be present in plasma of healthy humans and, in increased concentrations, in end-stage renal failure patients. The objectives of the study were to investigate the blood pressure and renal hemodynamic responses to Ang A in normotensive and hypertensive rats and in genetically modified mice and the binding properties of Ang A to Ang II type 1 (AT(1)) or Ang II type 2 (AT(2)) receptors. Intravenous and intrarenal administration of Ang A induced dose-dependent pressor and renal vasoconstrictor responses in normotensive rats, which were blocked by the AT(1) receptor antagonist candesartan but were not altered by the AT(2) receptor ligands PD123319, CGP42112A, or compound 21. Similar responses were observed after intravenous administration in spontaneously hypertensive rats. Deletion of AT(1a) receptors in mice almost completely abolished the pressor and renal vasoconstrictor responses to Ang A, indicating that its effects are mediated via AT(1a) receptors. Ang A was less potent than Ang II in vivo. The in vitro study demonstrated that Ang A is a full agonist for AT(1) receptors, with similar affinity for AT(1) and AT(2) receptors as Ang II. Overall, the responses to Ang A and Ang II were similar. Ang A has no physiological role to modulate the pressor and renal hemodynamic effects of Ang II.


