AZ 628Raf kinases,potent and ATP-competitive CAS# 878739-06-1 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
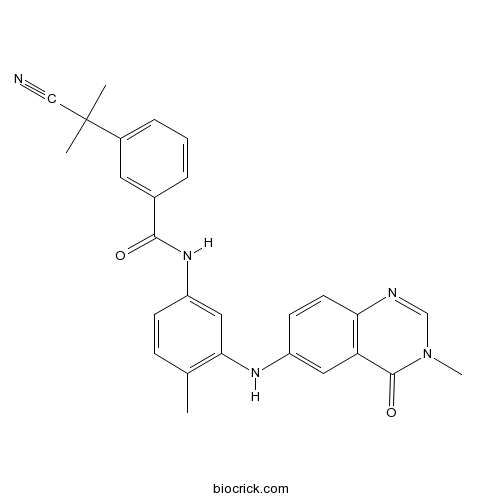
| Cas No. | 878739-06-1 | SDF | Download SDF |
| PubChem ID | 11676786 | Appearance | Powder |
| Formula | C27H25N5O2 | M.Wt | 451.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (110.74 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 3-(2-cyanopropan-2-yl)-N-[4-methyl-3-[(3-methyl-4-oxoquinazolin-6-yl)amino]phenyl]benzamide | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)C2=CC(=CC=C2)C(C)(C)C#N)NC3=CC4=C(C=C3)N=CN(C4=O)C | ||
| Standard InChIKey | ZGBGPEDJXCYQPH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H25N5O2/c1-17-8-9-21(31-25(33)18-6-5-7-19(12-18)27(2,3)15-28)14-24(17)30-20-10-11-23-22(13-20)26(34)32(4)16-29-23/h5-14,16,30H,1-4H3,(H,31,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, ATP-competitive inhibitor of Raf kinases (IC50 values are 29, 34 and 105 nM for c-Raf1, B-RafV600E and wild-type B-Raf, respectively). Displays selectivity for Raf kinases over a panel of 150 other kinases; inhibits activation of tyrosine protein kinases such as VEGFR2, Lyn, Flt1 and Fms. Also inhibits growth, and induces cell cycle arrest and apoptosis in colon and melanoma cell lines with the B-RafV600E mutation. |

AZ 628 Dilution Calculator

AZ 628 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2147 mL | 11.0735 mL | 22.1469 mL | 44.2938 mL | 55.3673 mL |
| 5 mM | 0.4429 mL | 2.2147 mL | 4.4294 mL | 8.8588 mL | 11.0735 mL |
| 10 mM | 0.2215 mL | 1.1073 mL | 2.2147 mL | 4.4294 mL | 5.5367 mL |
| 50 mM | 0.0443 mL | 0.2215 mL | 0.4429 mL | 0.8859 mL | 1.1073 mL |
| 100 mM | 0.0221 mL | 0.1107 mL | 0.2215 mL | 0.4429 mL | 0.5537 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AZ628 is a potent and newly discorvered inhibitor of BRAF, c-Raf-1 and BRAFV600E with IC50 values of 105 nM, 29 nM and 34 nM, respectively. This compound prevents CRAF activation through persistently occupying the ATP-binding site of Raf kinase. Specificity profile suggests that AZ628 also inhibits activation of other tyrosine protein kinases such as DDR2, VEGFR2, Lyn, Flt1, FMS and others.
Raf kinases a family of three serine/threonine-specific protein kinases and participate in the RAS-RAF-MEK-ERK signal transduction cascade, also known as the mitogen-activated protein kinase (MAPK) cascade. The activation of MAPK signaling leads to different cellular response such as cell proliferation, apoptosis and inflammation.
AZ628 has the potent anti-tumor activity. In human colon and melanoma-derived cell line that carries the recurrent V600E activating BRAF mutation, AZ628 was shown to inhibit anchorage-dependent and -independent growth, induce cell cycle arrest, and cause apoptosis [1]. AZ628 may be antiangiogenic due to inhibition of VEGFR2 [2].
Generation of melanoma cell line clones is obtained resistance to the RAF kinase inhibitor AZ628. Resistance to AZ628 is connected with raised levels of the RAF downstream effector p-ERK1/2. ERK1/2 initiation in AZ628-resistant clones is interceded by MEK. Supported multiplication of AZ628-resistant clones is to a great extent autonomous of BRAF kinase action. AZ628-resistant clones express elevated CRAF. Survival of AZ628-safe cells is subject to CRAF [1].
References:
1. Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 2008,68:4853-4861.
2. Khazak V, Astsaturov I, Serebriiskii IG, Golemis EA. Selective Raf inhibition in cancer therapy. Expert Opin Ther Targets 2007,11:1587-1609.
- Lesinurad
Catalog No.:BCC1699
CAS No.:878672-00-5
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
- WRW4
Catalog No.:BCC5893
CAS No.:878557-55-2
- JNJ 303
Catalog No.:BCC7806
CAS No.:878489-28-2
- Isosalviamine B
Catalog No.:BCN3554
CAS No.:878475-30-0
- Isosalviamine A
Catalog No.:BCN3553
CAS No.:878475-29-7
- Walrycin B
Catalog No.:BCC5156
CAS No.:878419-78-4
- Alismol
Catalog No.:BCN4427
CAS No.:87827-55-2
- S1RA
Catalog No.:BCC4189
CAS No.:878141-96-9
- Glucagon-like peptide 1 (1-37) (human, rat)
Catalog No.:BCC5827
CAS No.:87805-34-3
- 6beta-Hydroxyipolamiide
Catalog No.:BCN4426
CAS No.:87797-84-0
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
- 15-deoxy-Δ-12,14-Prostaglandin J2
Catalog No.:BCC7321
CAS No.:87893-55-8
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
- CP 93129 dihydrochloride
Catalog No.:BCC6899
CAS No.:879089-64-2
- Psoracorylifol A
Catalog No.:BCN3611
CAS No.:879290-97-8
- Psoracorylifol B
Catalog No.:BCN7884
CAS No.:879290-98-9
- Psoracorylifol C
Catalog No.:BCN3708
CAS No.:879290-99-0
- 3,4,5-Tri-O-galloylquinic acid
Catalog No.:BCN8027
CAS No.:99745-62-7
- p-Hydroxyphenethyl anisate
Catalog No.:BCN6896
CAS No.:87932-34-1
- Prionoid B
Catalog No.:BCN3217
CAS No.:879324-75-1
- Prionoid C
Catalog No.:BCN3159
CAS No.:879324-76-2
- Prionoid D
Catalog No.:BCN3160
CAS No.:879324-77-3
- Prionoid E
Catalog No.:BCN3161
CAS No.:879324-78-4
Highly potent and selective 3-N-methylquinazoline-4(3H)-one based inhibitors of B-Raf(V600E) kinase.[Pubmed:24675381]
Bioorg Med Chem Lett. 2014 Apr 15;24(8):1923-7.
Herein we describe the design of a novel series of ATP competitive B-Raf inhibitors via structure-based methods. These 3-N-methylquinazoline-4(3H)-one based inhibitors exhibit both excellent cellular potency and striking B-Raf selectivity. Optimization led to the identification of compound 16, a potent, selective and orally available agent with excellent pharmacokinetic properties and robust tumor growth inhibition in xenograft studies. Our work also demonstrates that by replacing an aryl amide with an aryl sulfonamide, a multikinase inhibitor such as AZ-628, can be converted to a selective B-Raf inhibitor, a finding that should have broad application in kinase drug discovery.
BAY61-3606 affects the viability of colon cancer cells in a genotype-directed manner.[Pubmed:22815993]
PLoS One. 2012;7(7):e41343.
BACKGROUND: K-RAS mutation poses a particularly difficult problem for cancer therapy. Activating mutations in K-RAS are common in cancers of the lung, pancreas, and colon and are associated with poor response to therapy. As such, targeted therapies that abrogate K-RAS-induced oncogenicity would be of tremendous value. METHODS: We searched for small molecule kinase inhibitors that preferentially affect the growth of colorectal cancer cells expressing mutant K-RAS. The mechanism of action of one inhibitor was explored using chemical and genetic approaches. RESULTS: We identified BAY61-3606 as an inhibitor of proliferation in colorectal cancer cells expressing mutant forms of K-RAS, but not in isogenic cells expressing wild-type K-RAS. In addition to its anti-proliferative effects in mutant cells, BAY61-3606 exhibited a distinct biological property in wild-type cells in that it conferred sensitivity to inhibition of RAF. In this context, BAY61-3606 acted by inhibiting MAP4K2 (GCK), which normally activates NFkappabeta signaling in wild-type cells in response to inhibition of RAF. As a result of MAP4K2 inhibition, wild-type cells became sensitive to AZ-628, a RAF inhibitor, when also treated with BAY61-3606. CONCLUSIONS: These studies indicate that BAY61-3606 exerts distinct biological activities in different genetic contexts.
The role of hydrogen bonding at the active site of a cupredoxin: the Phe114Pro azurin variant.[Pubmed:16846224]
Biochemistry. 2006 Jul 25;45(29):8812-22.
The Phe114Pro mutation to the cupredoxin azurin (AZ) leads to a number of structural changes at the active site attributed to deletion of one of the hydrogen bonds to the Cys112 ligand, removal of the bulky phenyl group from the hydrophobic patch of the protein, and steric interactions made by the introduced Pro. The remaining hydrogen bond between the coordinating thiolate and the backbone amide of Asn47 is strengthened. At the type-1 copper site, the Cu(II)-O(Gly45) axial interaction decreases, while the metal moves out of the plane formed by the equatorial His46, Cys112, and His117 ligands, shortening the bond to the axially coordinating Met121. The resulting distorted tetrahedral geometry is distinct from the trigonal bipyramidal arrangement in the wild-type (WT) protein. The unique position of the main S(Cys) --> Cu(II) ligand-to-metal charge-transfer transition in AZ (628 nm) has shifted in the Phe114Pro variant to a value that is more typical for cupredoxins (599 nm). This probably occurs because of the removal of the Phe114-Cys112 hydrogen bond. The Phe114Pro mutation results in a 90 mV decrease in the reduction potential of AZ, and removal of the second hydrogen bond to the Cys ligand seems to be the major cause of this change. The C-terminal His117 ligand does not protonate in the reduced Phe114Pro AZ variant, which suggests that none of the structural features altered by the mutation are responsible for the absence of this effect in the WT protein. Upon reduction, the copper displaces further from the equatorial ligand plane and the Cu-S(Met121) bond length decreases. These changes are larger than those seen in the WT protein and contribute to the order of magnitude decrease in the intrinsic electron-transfer capabilities of the Phe114Pro variant.
RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth.[Pubmed:20130576]
Nature. 2010 Mar 18;464(7287):431-5.
Activating mutations in KRAS and BRAF are found in more than 30% of all human tumours and 40% of melanoma, respectively, thus targeting this pathway could have broad therapeutic effects. Small molecule ATP-competitive RAF kinase inhibitors have potent antitumour effects on mutant BRAF(V600E) tumours but, in contrast to mitogen-activated protein kinase kinase (MEK) inhibitors, are not potent against RAS mutant tumour models, despite RAF functioning as a key effector downstream of RAS and upstream of MEK. Here we show that ATP-competitive RAF inhibitors have two opposing mechanisms of action depending on the cellular context. In BRAF(V600E) tumours, RAF inhibitors effectively block the mitogen-activated protein kinase (MAPK) signalling pathway and decrease tumour growth. Notably, in KRAS mutant and RAS/RAF wild-type tumours, RAF inhibitors activate the RAF-MEK-ERK pathway in a RAS-dependent manner, thus enhancing tumour growth in some xenograft models. Inhibitor binding activates wild-type RAF isoforms by inducing dimerization, membrane localization and interaction with RAS-GTP. These events occur independently of kinase inhibition and are, instead, linked to direct conformational effects of inhibitors on the RAF kinase domain. On the basis of these findings, we demonstrate that ATP-competitive kinase inhibitors can have opposing functions as inhibitors or activators of signalling pathways, depending on the cellular context. Furthermore, this work provides new insights into the therapeutic use of ATP-competitive RAF inhibitors.
Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma.[Pubmed:18559533]
Cancer Res. 2008 Jun 15;68(12):4853-61.
Activating BRAF kinase mutations arise in approximately 7% of all human tumors, and preclinical studies have validated the RAF-mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase-ERK signaling cascade as a potentially important therapeutic target in this setting. Selective RAF kinase inhibitors are currently undergoing clinical development, and based on the experience with other kinase-targeted therapeutics, it is expected that clinical responses to these agents, if observed, will lead to the eventual emergence of drug resistance in most cases. Thus, it is important to establish molecular mechanisms underlying such resistance to develop effective therapeutic strategies to overcome or prevent drug resistance. To anticipate potential mechanisms of acquired resistance to RAF inhibitors during the course of treatment, we established drug-resistant clones from a human melanoma-derived cell line harboring the recurrent V600E activating BRAF mutation, which exhibits exquisite sensitivity to AZ628, a selective RAF kinase inhibitor. We determined that elevated CRAF protein levels account for the acquisition of resistance to AZ628 in these cells, associated with a switch from BRAF to CRAF dependency in tumor cells. We also found that elevated CRAF protein levels may similarly contribute to primary insensitivity to RAF inhibition in a subset of BRAF mutant tumor cells. Interestingly, AZ628-resistant cells demonstrating either primary drug insensitivity or acquired drug resistance exhibit exquisite sensitivity to the HSP90 inhibitor geldanamycin. Geldanamycin effectively promotes the degradation of CRAF, thereby revealing a potential therapeutic strategy to overcome resistance to RAF inhibition in a subset of BRAF mutant tumors.
Selective Raf inhibition in cancer therapy.[Pubmed:18020980]
Expert Opin Ther Targets. 2007 Dec;11(12):1587-609.
Over the past 5 years, the Raf kinase family has emerged as a promising target for protein-directed cancer therapy development. The goal of this review is to first provide a concise summary of the data validating Raf proteins as high-interest therapeutic targets. The authors then outline the mode of action of Raf kinases, emphasizing how Raf activities and protein interactions suggest specific approaches to inhibiting Raf. The authors then summarize the set of drugs, antisense reagents and antibodies available or in development for therapeutically targeting Raf or Raf-related proteins, as well as existing strategies combining these and other therapeutic agents. Finally, the authors discuss recent results from systems biology analyses that have the potential to increasingly guide the intelligent selection of combination therapies involving Raf-targeting agents and other therapeutics.


