AC 55541PAR2 agonist,potent and selective CAS# 916170-19-9 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 916170-19-9 | SDF | Download SDF |
| PubChem ID | 9589606 | Appearance | Powder |
| Formula | C25H20BrN5O3 | M.Wt | 518.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 51 mg/mL (98.39 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
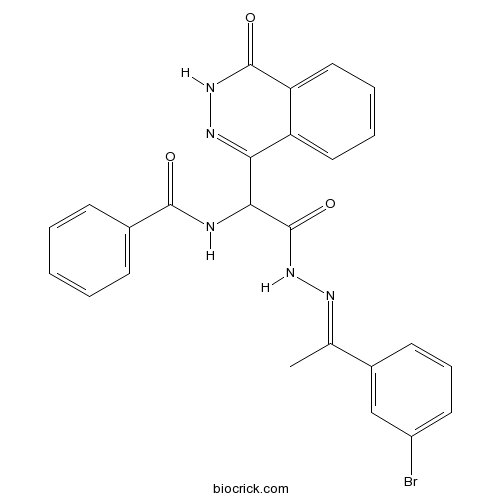
| Chemical Name | N-[2-[(2E)-2-[1-(3-bromophenyl)ethylidene]hydrazinyl]-2-oxo-1-(4-oxo-3H-phthalazin-1-yl)ethyl]benzamide | ||
| SMILES | CC(=NNC(=O)C(C1=NNC(=O)C2=CC=CC=C21)NC(=O)C3=CC=CC=C3)C4=CC(=CC=C4)Br | ||
| Standard InChIKey | UCUHFWIFSHROPY-RWPZCVJISA-N | ||
| Standard InChI | InChI=1S/C25H20BrN5O3/c1-15(17-10-7-11-18(26)14-17)28-31-25(34)22(27-23(32)16-8-3-2-4-9-16)21-19-12-5-6-13-20(19)24(33)30-29-21/h2-14,22H,1H3,(H,27,32)(H,30,33)(H,31,34)/b28-15+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective protease-activated receptor 2 (PAR2) agonist (pEC50 = 6.7) that displays no activity at other PAR subtypes or at over 30 other receptors involved in nociception and inflammation. Stimulates cell proliferation, PI hydrolysis and Ca2+ mobilization in vitro (pEC50 values are 6.7, 5.9 and 6.6 respectively) and exhibits pronociceptive activity in vivo. |

AC 55541 Dilution Calculator

AC 55541 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9292 mL | 9.6458 mL | 19.2916 mL | 38.5832 mL | 48.229 mL |
| 5 mM | 0.3858 mL | 1.9292 mL | 3.8583 mL | 7.7166 mL | 9.6458 mL |
| 10 mM | 0.1929 mL | 0.9646 mL | 1.9292 mL | 3.8583 mL | 4.8229 mL |
| 50 mM | 0.0386 mL | 0.1929 mL | 0.3858 mL | 0.7717 mL | 0.9646 mL |
| 100 mM | 0.0193 mL | 0.0965 mL | 0.1929 mL | 0.3858 mL | 0.4823 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AC 55541 is a potent agonist of PAR2 with pEC50 value of 6.7 and functions range from 200 to 1000 nM [1-3].
Protease-activated receptor 2 (PAR2) is a member of the large family of 7-transmembrane receptors and plays an important role in modulating inflammatory responses and acting as a sensor for proteolytic enzymes generated during infection [2].
AC 55541 is a selective PAR2 agonist and has no effect on PAR1. Using a cellular proliferation assay (R-SAT), it showed that AC 55541 stimulated NIH-3T3 cells proliferation by activating PAR2 and receptor with F240S mutation constitutively active in all functional assays which nearly 30-fold sensitive to AC 55541 than SLIGRL and 2-furorl LIGRLO [1, 2]. When tested with human corneal epithelial (HCE) cells, administration of AC 55541 significantly induced the expression of PAR2 and inhibited Acanthamoeba plasminogen activator (aPA) [3].
In male Sprague-Dawley rat model with acute thermal nociception and edema in paw induced by SLIGRL-NH2 or trypsin, intrapaw administration of AC 55541 induced hind paw edema and thermal hyperalgesia at the doses as low as 30 ng and showed dose-dependent pronociceptive effects [1].
References:
1. Gardell, L.R., et al., Identification and characterization of novel small-molecule protease-activated receptor 2 agonists. J Pharmacol Exp Ther, 2008. 327(3): p. 799-808.
2. Ma, J.N. and E.S. Burstein, The protease activated receptor 2 (PAR2) polymorphic variant F240S constitutively activates PAR2 receptors and potentiates responses to small-molecule PAR2 agonists. J Pharmacol Exp Ther, 2013. 347(3): p. 697-704.
3. Tripathi, T., M. Abdi, and H. Alizadeh, Protease-activated receptor 2 (PAR2) is upregulated by Acanthamoeba plasminogen activator (aPA) and induces proinflammatory cytokine in human corneal epithelial cells. Invest Ophthalmol Vis Sci, 2014. 55(6): p. 3912-21.
- TCS 1102
Catalog No.:BCC4063
CAS No.:916141-36-1
- Benidipine HCl
Catalog No.:BCC4395
CAS No.:91599-74-5
- Momordicine I
Catalog No.:BCN3058
CAS No.:91590-76-0
- Dovitinib (TKI258) Lactate
Catalog No.:BCC6473
CAS No.:915769-50-5
- WAY 316606
Catalog No.:BCC2052
CAS No.:915759-45-4
- ABC294640
Catalog No.:BCC4192
CAS No.:915385-81-8
- Cot inhibitor-1
Catalog No.:BCC1496
CAS No.:915365-57-0
- Cot inhibitor-2
Catalog No.:BCC1497
CAS No.:915363-56-3
- Bakkenolide IIIa
Catalog No.:BCN7352
CAS No.:915289-60-0
- 3-[4-[(2-Chloro-5-iodophenyl)methyl]phenoxy]tetrahydro-furan
Catalog No.:BCC8601
CAS No.:915095-94-2
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
- BEZ235 (NVP-BEZ235)
Catalog No.:BCC3655
CAS No.:915019-65-7
- Gopherenediol
Catalog No.:BCN6582
CAS No.:916236-79-8
- Acetylvirolin
Catalog No.:BCN7041
CAS No.:916264-22-7
- Clinodiside A
Catalog No.:BCN1048
CAS No.:916347-31-4
- Senkyunolide C
Catalog No.:BCC9141
CAS No.:91652-78-7
- Zingiberen newsaponin
Catalog No.:BCN2942
CAS No.:91653-50-8
- Coriatin
Catalog No.:BCN4457
CAS No.:91653-75-7
- Clematomandshurica saponin B
Catalog No.:BCN7810
CAS No.:916649-91-7
- Clematiunicinoside E
Catalog No.:BCN7809
CAS No.:916649-92-8
- TC-I 15
Catalog No.:BCC6216
CAS No.:916734-43-5
- 5,7,4'-Trihydroxy-8-methylflavanone
Catalog No.:BCN2844
CAS No.:916917-28-7
- Enniatin B
Catalog No.:BCN4774
CAS No.:917-13-5
- 2-[(Acetylthio)methyl]-phenylpropionic acid
Catalog No.:BCC8507
CAS No.:91702-98-6
Reducing mass peak instability caused by the phase changes of RF and AC signals in a rectilinear ion-trap analyzer.[Pubmed:28372366]
Rev Sci Instrum. 2017 Mar;88(3):034103.
For an ion trap with resonance ejection, peak intensity and peak position of the acquired mass spectra are affected by the phase difference between the radio frequency (RF) and auxiliary alternating current (AC) potentials. To ensure measurement stability, RF and AC phase-locking is commonly used in commercial ion trap mass spectrometers. In this study, a compact electronic control system was developed to accurately regulate the RF and AC phases and was employed in a photoionization rectilinear ion trap (RIT) mass spectrometer. We found that the phase-locking method was defective in multicomponent analysis because the optimal RF and AC phase difference was usually different for different m/z peaks. After studying and characterizing the relationship between the peaks and the RF and AC phases, a correction method based on data processing was used to improve the peaks' stability and accuracy. The results show that the fluctuations of both peak intensity and peak position were significantly reduced and that the instrument presented satisfying reproducibility and quantitative ability.
Multi-isotope SPECT imaging of the (225)Ac decay chain: feasibility studies.[Pubmed:28362640]
Phys Med Biol. 2017 Jun 7;62(11):4406-4420.
Effective use of the [Formula: see text] decay chain in targeted internal radioimmunotherapy requires the retention of both [Formula: see text] and progeny isotopes at the target site. Imaging-based pharmacokinetic tests of these pharmaceuticals must therefore separately yet simultaneously image multiple isotopes that may not be colocalized despite being part of the same decay chain. This work presents feasibility studies demonstrating the ability of a microSPECT/CT scanner equipped with a high energy collimator to simultaneously image two components of the [Formula: see text] decay chain: [Formula: see text] (218 keV) and [Formula: see text] (440 keV). Image quality phantoms were used to assess the performance of two collimators for simultaneous [Formula: see text] and [Formula: see text] imaging in terms of contrast and noise. A hotrod resolution phantom containing clusters of thin rods with diameters ranging between 0.85 and 1.70 mm was used to assess resolution. To demonstrate ability to simultaneously image dynamic [Formula: see text] and [Formula: see text] activity distributions, a phantom containing a [Formula: see text] generator from [Formula: see text] was imaged. These tests were performed with two collimators, a high-energy ultra-high resolution (HEUHR) collimator and an ultra-high sensitivity (UHS) collimator. Values consistent with activity concentrations determined independently via gamma spectroscopy were observed in high activity regions of the images. In hotrod phantom images, the HEUHR collimator resolved all rods for both [Formula: see text] and [Formula: see text] images. With the UHS collimator, no rods were resolvable in [Formula: see text] images and only rods 1.3 mm were resolved in [Formula: see text] images. After eluting the [Formula: see text] generator, images accurately visualized the reestablishment of transient equilibrium of the [Formula: see text] decay chain. The feasibility of evaluating the pharmacokinetics of the [Formula: see text] decay chain in vivo has been demonstrated. This presented method requires the use of a high-performance high-energy collimator.
Response of phenolic metabolism to cadmium and phenanthrene and its influence on pollutant translocations in the mangrove plant Aegiceras corniculatum (L.) Blanco (Ac).[Pubmed:28363172]
Ecotoxicol Environ Saf. 2017 Jul;141:290-297.
Polyphenolic compounds are abundant in mangrove plants, playing a pivotal role in the detoxification of pollutants extruded from surrounding environments into plant tissues. The present study aimed to examine the variations of phenolic compounds, namely total polyphenolics, soluble tannins, condensed tannins and lignin, in the mangrove plant Aegiceras corniculatum (L.) due to the presence of exogenous cadmium and phenanthrene and to explore the influence of phenolic metabolism on biological translocation of these pollutants from roots to leaves. After a 6-week exposure to cadmium and phenanthrene, significant accumulations of both pollutants were observed. All determined phenolic compounds in both leaves and roots at high dosage levels were enhanced compared to the uncontaminated plant. Elevations of polyphenols in both treatments are possibly a result of stimulation in the activity of phenylalanine ammonia-lyase (PAL) and the enrichment of soluble sugar. Additionally, a significantly positive dosage relationship between polyphenolic metabolism intensity and phenanthrene contamination levels was found, while the trend observed in cadmium treatment was weak since cadmium at high levels inhibited phenolic production. The enrichment of polyphenols led to a decline in the biological translocation of these pollutants from roots to leaves. The immobilization of pollutants in the plant roots is possibly linked to the adsorption potential of polyphenols. These results will improve the understanding of the tolerance of mangrove plants to exogenous pollutants and will guide the selection of plants in phytoremediation because of the variability of polyphenol concentrations among species.
Investigation of ac-magnetic field stimulated nanoelectroporation of magneto-electric nano-drug-carrier inside CNS cells.[Pubmed:28374799]
Sci Rep. 2017 Apr 4;7:45663.
In this research, we demonstrate cell uptake of magneto-electric nanoparticles (MENPs) through nanoelectroporation (NEP) using alternating current (ac)-magnetic field stimulation. Uptake of MENPs was confirmed using focused-ion-beam assisted transmission electron microscopy (FIB-TEM) and validated by a numerical simulation model. The NEP was performed in microglial (MG) brain cells, which are highly sensitive for neuro-viral infection and were selected as target for nano-neuro-therapeutics. When the ac-magnetic field optimized (60 Oe at 1 kHz), MENPs were taken up by MG cells without affecting cell health (viability > 92%). FIB-TEM analysis of porated MG cells confirmed the non-agglomerated distribution of MENPs inside the cell and no loss of their elemental and crystalline characteristics. The presented NEP method can be adopted as a part of future nanotherapeutics and nanoneurosurgery strategies where a high uptake of a nanomedicine is required for effective and timely treatment of brain diseases.
Identification and characterization of novel small-molecule protease-activated receptor 2 agonists.[Pubmed:18768780]
J Pharmacol Exp Ther. 2008 Dec;327(3):799-808.
We report the first small-molecule protease-activated receptor (PAR) 2 agonists, AC-55541 [N-[[1-(3-bromo-phenyl)-eth-(E)-ylidene-hydrazinocarbonyl]-(4-oxo-3,4-dihydro-pht halazin-1-yl)-methyl]-benzamide] and AC-264613 [2-oxo-4-phenylpyrrolidine-3-carboxylic acid [1-(3-bromo-phenyl)-(E/Z)-ethylidene]-hydrazide], each representing a distinct chemical series. AC-55541 and AC-264613 each activated PAR2 signaling in cellular proliferation assays, phosphatidylinositol hydrolysis assays, and Ca(2+) mobilization assays, with potencies ranging from 200 to 1000 nM for AC-55541 and 30 to 100 nM for AC-264613. In comparison, the PAR2-activating peptide 2-furoyl-LIGRLO-NH(2) had similar potency, whereas SLIGRL-NH(2) was 30 to 300 times less potent. Neither AC-55541 nor AC-264613 had activity at any of the other PAR receptor subtypes, nor did they have any significant affinity for over 30 other molecular targets involved in nociception. Visualization of EYFP-tagged PAR2 receptors showed that each compound stimulated internalization of PAR2 receptors. AC-55541 and AC-264613 were well absorbed when administered intraperitoneally to rats, each reaching micromolar peak plasma concentrations. AC-55541 and AC-264613 were each stable to metabolism by liver microsomes and maintained sustained exposure in rats, with elimination half-lives of 6.1 and 2.5 h, respectively. Intrapaw administration of AC-55541 or AC-264613 elicited robust and persistent thermal hyperalgesia and edema. Coadministration of either a tachykinin 1 (neurokinin 1) receptor antagonist or a transient receptor potential vanilloid (TRPV) 1 antagonist completely blocked these effects. Systemic administration of either AC-55541 or AC-264613 produced a similar degree of hyperalgesia as was observed when the compounds were administered locally. These compounds represent novel small-molecule PAR2 agonists that will be useful in probing the physiological functions of PAR2 receptors.
Discovery of potent and selective small-molecule PAR-2 agonists.[Pubmed:18720984]
J Med Chem. 2008 Sep 25;51(18):5490-3.
Proteinase activated receptor-2 plays a crucial role in a wide variety of conditions with a strong inflammatory component. We present the discovery and characterization of two structurally different, potent, selective, and metabolically stable small-molecule PAR-2 agonists. These ligands may be useful as pharmacological tools for elucidating the complex physiological role of the PAR-2 receptors as well as for the development of PAR-2 antagonists.


