5-AzacytidineDNA methyltransferase inhibitor. CAS# 320-67-2 |
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
Number of papers citing our products

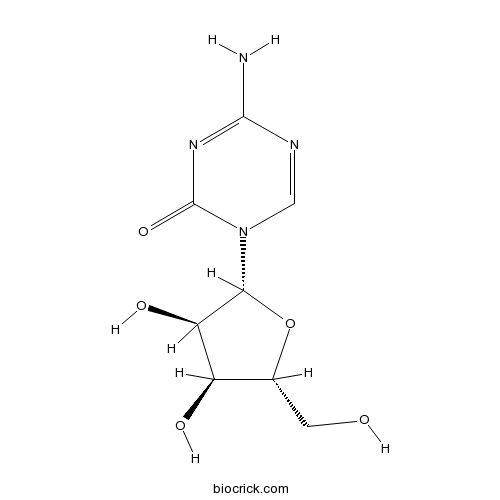
Chemical structure
3D structure
| Cas No. | 320-67-2 | SDF | Download SDF |
| PubChem ID | 9444 | Appearance | Powder |
| Formula | C8H12N4O5 | M.Wt | 244.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ladakamycin; 5-AzaC; Azacitidine | ||
| Solubility | DMSO : ≥ 31 mg/mL (126.95 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one | ||
| SMILES | C1=NC(=NC(=O)N1C2C(C(C(O2)CO)O)O)N | ||
| Standard InChIKey | NMUSYJAQQFHJEW-KVTDHHQDSA-N | ||
| Standard InChI | InChI=1S/C8H12N4O5/c9-7-10-2-12(8(16)11-7)6-5(15)4(14)3(1-13)17-6/h2-6,13-15H,1H2,(H2,9,11,16)/t3-,4-,5-,6-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-Azacytidine is a nucleoside analogue of cytidine that specifically inhibits DNA methylation by trapping DNA methyltransferases. |

5-Azacytidine Dilution Calculator

5-Azacytidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.095 mL | 20.475 mL | 40.95 mL | 81.9001 mL | 102.3751 mL |
| 5 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 10 mM | 0.4095 mL | 2.0475 mL | 4.095 mL | 8.19 mL | 10.2375 mL |
| 50 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| 100 mM | 0.041 mL | 0.2048 mL | 0.4095 mL | 0.819 mL | 1.0238 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
5-Azacytidine (also known as 5-AzaC), a compound belonging to a class of cytosine analogues, is a DNA methyl transferase (DNMT) inhibitor that exerts potent cytotoxicity against multiple myeloma (MM) cells, including MM.1S, MM.1R, RPMI-8266, RPMI-LR5, RPMI-Dox40 and Patient-derived MM, with the half maximal inhibition concentration IC50 values of 1.5 μmol/L, 0.7 μmol/L, 1.1 μmol/L, 2.5 μmol/L, 3.2 μmol/L and 1.5 μmol/L respectively [1].
5-Azacytidine incorporates into cellular DNA and/or RNA, subsequently sequesters DNMT and forms a covalent bond between C6 of 5-Azacytidine and cysteine thiolate of DNMTs resulting in depletion of DNMT activity in cells and demethylation of cellular DNA [1].
References:
[1] Kiziltepe T, Hideshima T, Catley L, Raje N, Yasui H, Shiraishi N, Okawa Y, Ikeda H, Vallet S, Pozzi S, Ishitsuka K, Ocio EM, Chauhan D, Anderson KC. 5-Azacytidine, a DNA methyltransferase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol Cancer Ther. 2007 Jun;6(6):1718-27.
- SIB 1757
Catalog No.:BCC6971
CAS No.:31993-01-8
- Ethyl glucoside
Catalog No.:BCN5239
CAS No.:3198-49-0
- H-β-Ala-OMe.HCl
Catalog No.:BCC2853
CAS No.:3196-73-4
- Norarmepavine
Catalog No.:BCN7077
CAS No.:3195-01-5
- Cornucervine
Catalog No.:BCN1962
CAS No.:31948-48-8
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- (RS)-3-Hydroxyphenylglycine
Catalog No.:BCC6604
CAS No.:31932-87-3
- H-D-Allo-Ile-OH
Catalog No.:BCC2683
CAS No.:319-78-8
- Moricizine
Catalog No.:BCC5235
CAS No.:31883-05-3
- Methyl orsellinate
Catalog No.:BCN5238
CAS No.:3187-58-4
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
- Aclidinium Bromide
Catalog No.:BCC4575
CAS No.:320345-99-1
- Marmin acetonide
Catalog No.:BCN5240
CAS No.:320624-68-8
- Adenine sulfate
Catalog No.:BCC4451
CAS No.:321-30-2
- Fluoronaphthalene
Catalog No.:BCC8987
CAS No.:321-38-0
- EO 1428
Catalog No.:BCC7511
CAS No.:321351-00-2
- [D-Trp8]-γ-MSH
Catalog No.:BCC7902
CAS No.:321351-81-9
- Bellendine
Catalog No.:BCN1895
CAS No.:32152-73-1
- Z- Pyr-OH
Catalog No.:BCC3330
CAS No.:32159-21-0
- N-Acetyl-4-piperidone
Catalog No.:BCC9079
CAS No.:32161-06-1
- Cytosporone B
Catalog No.:BCN6791
CAS No.:321661-62-5
- BIBR 1532
Catalog No.:BCC1147
CAS No.:321674-73-1
- Poloxin
Catalog No.:BCC1867
CAS No.:321688-88-4
Modifying effects of 5-azacytidine on metal-containing proteins profile in Guerin carcinoma with different sensitivity to cytostatics.[Pubmed:28230826]
Exp Oncol. 2016 Dec;38(4):283-287.
AIM: To assess the influence of the treatment with 5-Azacytidine (5-aza) on the profile of metal-containing proteins and factors of their regulation in Guerin carcinoma cells in vivo. MATERIALS AND METHODS: The study was conducted on Wistar rats transplanted with wild-type Guerin carcinoma (Guerin/WT) and its strains resistant to cisplatin (Guerin/CP) or doxorubicin (Guerin/Dox). Animals were distributed in 6 groups treated with 5-aza and control animals without treatment. 5-Aza was injected by i.v. route (1 injection in 4 days at a dose of 2 mg/kg starting from the 4(th) day after tumor transplantation, 4 injections in total). Ferritin levels in blood serum and tumor tissue were measured by ELISA, transferrin and free iron complexes - by low-temperature EPR, miRNA-200b, -133a and -320a levels and promoter methylation - by real-time quantitative reverse transcription polymerase chain reaction. RESULTS: The study has shown that 5-aza treatment caused demethylation of promoter regions of fth1 and tfr1 genes in all studied Guerin carcinoma strains. 5-Aza treatment resulted in a significant decrease of ferritin levels in tumor tissue (by 32.1% in Guerin/WT strain, by 29.8% in Guerin/Dox and by 69.1% in Guerin/CP). These events were accompanied by 3.5-fold and 2-fold increase of free iron complexes levels in tumor tissue of doxorubicin and cisplatin resistant strains, respectively. Also, 5-aza treatment resulted in significantly elevated levels of miR-200b, -133a, 320a expression in tumor tissue. After 5-aza treatment, ferritin levels in blood serum of animals with Guerin/Dox were increased by 23.9%, while in Guerin/Wt and Guerin/CP they were decreased by 17 and 16%, respectively. CONCLUSION: Alterations of epigenetic regulation upon in vivo treatment with 5-aza change the levels of metal-containing proteins due to DNA demethylation and altered miRNA expression profiles in Guerin carcinoma cells.
An Improved Syringe Agroinfiltration Protocol to Enhance Transformation Efficiency by Combinative Use of 5-Azacytidine, Ascorbate Acid and Tween-20.[Pubmed:28216553]
Plants (Basel). 2017 Feb 14;6(1). pii: plants6010009.
Syringe infiltration is an important transient transformation method that is widely used in many molecular studies. Owing to the wide use of syringe agroinfiltration, it is important and necessary to improve its transformation efficiency. Here, we studied the factors influencing the transformation efficiency of syringe agroinfiltration. The pCAMBIA1301 was transformed into Nicotiana benthamiana leaves for investigation. The effects of 5-Azacytidine (AzaC), Ascorbate acid (ASC) and Tween-20 on transformation were studied. The beta-glucuronidase (GUS) expression and GUS activity were respectively measured to determine the transformation efficiency. AzaC, ASC and Tween-20 all significantly affected the transformation efficiency of agroinfiltration, and the optimal concentrations of AzaC, ASC and Tween-20 for the transgene expression were identified. Our results showed that 20 muM AzaC, 0.56 mM ASC and 0.03% (v/v) Tween-20 is the optimal concentration that could significantly improve the transformation efficiency of agroinfiltration. Furthermore, a combined supplement of 20 muM AzaC, 0.56 mM ASC and 0.03% Tween-20 improves the expression of transgene better than any one factor alone, increasing the transgene expression by more than 6-fold. Thus, an optimized syringe agroinfiltration was developed here, which might be a powerful method in transient transformation analysis.
How to Diagnose Early 5-Azacytidine-Induced Pneumonitis: A Case Report.[Pubmed:28217822]
Drug Saf Case Rep. 2017 Dec;4(1):4.
Interstitial pneumonitis is a classical complication of many drugs. Pulmonary toxicity due to 5-Azacytidine, a deoxyribonucleic acid methyltransferase inhibitor and cytotoxic drug, has rarely been reported. We report a 67-year-old female myelodysplastic syndrome patient treated with 5-Azacytidine at the conventional dosage of 75 mg/m(2) for 7 days. One week after starting she developed moderate fever along with dry cough and subsequently her temperature rose to 39.5 degrees C. She was placed under broad-spectrum antibiotics based on the protocol for febrile neutropenia, including ciprofloxacin 750 mg twice daily, ceftazidime 1 g three times daily (tid), and sulfamethoxazole/trimethoprim 400 mg/80 mg tid. High-resolution computed tomography of the chest disclosed diffuse bilateral opacities with ground-glass shadowing and pleural effusion bilaterally. Mediastinal and hilar lymph nodes were moderately enlarged. polymerase chain reaction for Mycobacterium tuberculosis, Pneumocystis jiroveci, and cytomegalovirus were negative. Cultures including viral and fungal were all negative. A diagnosis of drug-induced pneumonitis was considered and, given the negative bronchoalveolar lavage in terms of an infection, corticosteroid therapy was given at a dose of 1 mg/kg body weight. Within 4 weeks, the patient became afebrile and was discharged from hospital. Development of symptoms with respect to drug administration, unexplained fever, negative workup for an infection, and marked response to corticosteroid therapy were found in our case. An explanation could be a delayed type of hypersensitivity (type IV) with activation of CD8 T cell which could possibly explain most of the symptoms. We have developed a decision algorithm in order to anticipate timely diagnosis of 5-azacitidine-induced pneumonitis, and with the aim to limit antibiotics abuse and to set up emergency treatment.
5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase.[Pubmed:21476855]
Stem Cells Dev. 2012 Jan;21(1):67-75.
5-Azacytidine (5-Aza) induces differentiation of mesenchymal stem cells (MSCs) into cardiomyocytes. However, the underlying mechanisms are not well understood. Our previous work showed that 5-Aza induces human bone marrow-derived MSCs to differentiate into cardiomyocytes. Here, we demonstrated that 5-Aza induced cardiac differentiation of human umbilical cord-derived MSCs (hucMSCs) and explored the potential signaling pathway. Our results showed that hucMSCs had cardiomyocyte phenotypes after 5-Aza treatment. In addition, myogenic cells differentiated from hucMSCs were positive for mRNA and protein of desmin, beta-myosin heavy chain, cardiac troponin T, A-type natriuretic peptide, and Nkx2.5. Human diploid lung fibroblasts treated with 5-Aza expressed no cardiac-specific genes. 5-Aza did not induce hucMSCs to differentiate into osteoblasts. Further study revealed that 5-Aza treatment activated extracellular signal related kinases (ERK) in hucMSCs, but protein kinase C showed no response to 5-Aza administration. U0126, a specific inhibitor of ERK, could inhibit 5-Aza-induced expression of cardiac-specific genes and proteins in hucMSCs. Increased phosphorylation of signal transducers and activators of transcription 3, and up-regulation of myocyte enhancer-binding factor-2c and myogenic differentiation antigen in 5-Aza-treated hucMSCs were also suppressed by U0126. Taken together, these results suggested that sustained activation of ERK by 5-Aza contributed to the induction of the differentiation of hucMSCs into cardiomyocytes in vitro.
Dissecting direct reprogramming through integrative genomic analysis.[Pubmed:18509334]
Nature. 2008 Jul 3;454(7200):49-55.
Somatic cells can be reprogrammed to a pluripotent state through the ectopic expression of defined transcription factors. Understanding the mechanism and kinetics of this transformation may shed light on the nature of developmental potency and suggest strategies with improved efficiency or safety. Here we report an integrative genomic analysis of reprogramming of mouse fibroblasts and B lymphocytes. Lineage-committed cells show a complex response to the ectopic expression involving induction of genes downstream of individual reprogramming factors. Fully reprogrammed cells show gene expression and epigenetic states that are highly similar to embryonic stem cells. In contrast, stable partially reprogrammed cell lines show reactivation of a distinctive subset of stem-cell-related genes, incomplete repression of lineage-specifying transcription factors, and DNA hypermethylation at pluripotency-related loci. These observations suggest that some cells may become trapped in partially reprogrammed states owing to incomplete repression of transcription factors, and that DNA de-methylation is an inefficient step in the transition to pluripotency. We demonstrate that RNA inhibition of transcription factors can facilitate reprogramming, and that treatment with DNA methyltransferase inhibitors can improve the overall efficiency of the reprogramming process.
5-Aza-cytidine is a potent inhibitor of DNA methyltransferase 3a and induces apoptosis in HCT-116 colon cancer cells via Gadd45- and p53-dependent mechanisms.[Pubmed:15547111]
J Pharmacol Exp Ther. 2005 Feb;312(2):525-36.
Methyltransferase inhibitors commonly used in clinical trials promote tumor cell death, but their detailed cytotoxic action is not yet fully understood. A deeper knowledge about their apotosis-inducing mechanisms and their interaction with DNA methyltransferases (DNMTs) DNMT1, DNMT3a, and DNMT3b might allow the design of more effective drugs with lower cytotoxicity. 5-aza-cytidine (5-aza-CR), a potent inhibitor of DNMT1, is known to induce demethylation and reactivation of silenced genes. In this study, we investigated the p53 dependence of apoptotic, cell cycle, and growth inhibitory effects of 5-aza-CR, as well as the influence on the expression level of DNMT1, DNMT3a, and DNMT3b in the colon cancer cell line HCT-116. Exposure to 5-aza-CR induced the up-regulation of genes promoting cell cycle arrest and DNA repair (p21(WAF1) and GADD45) or apoptosis (p53, RIPK2, Bak1, caspase 5, and caspase 6). In parallel, there was a down-regulation of antiapoptotic Bcl2 protein and the G(2)/M-mediator cyclin B1. Co-incubation with pifithrin-alpha (PFT-alpha), a selective p53 inhibitor, restored GADD45, Bcl2, cyclin B1, and p21(WAF1) expression levels and almost completely reversed the growth inhibitory, cell cycle, and apoptotic effects of 5-aza-CR. 5-aza-CR treatment caused global demethylation and reactivation of p16(INK4) expression. There was a marked decrease in DNMT1 and DNMT3a mRNA expression, with PFT-alpha reversing these effects. However, 5-aza-CR treatment did not modulate DNMT3b expression. Our data demonstrate that 5-aza-CR action in HCT-116 is mediated by p53 and its downstream effectors p21(WAF1) and GADD45. This is the first report to show a link between p53 and regulation of DNMT1 and de novo methyltransferase DNMT3a.


