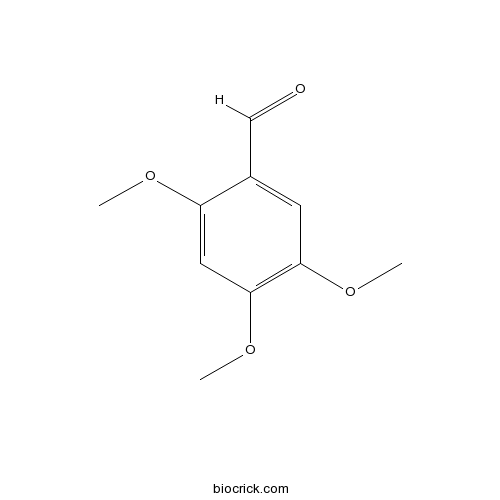
2,4,5-TrimethoxybenzaldehydeCAS# 4460-86-0 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 4460-86-0 | SDF | Download SDF |
| PubChem ID | 20525 | Appearance | Yellow powder |
| Formula | C10H12O4 | M.Wt | 196.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Asaronaldehyde; 14374-62-0;Asaraldehyde; 2,4,5-trimethoxy-Benzaldehyde | ||
| Solubility | H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2,4,5-trimethoxybenzaldehyde | ||
| SMILES | COC1=CC(=C(C=C1C=O)OC)OC | ||
| Standard InChIKey | IAJBQAYHSQIQRE-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2,4,5-Trimethoxybenzaldehyde (2,4,5-TMBA) has anti-adipogenic potential, is a natural cyclooxygenase II (COX-2) inhibitor, suppresses the differentiation of preadipocyts into adipocytes at the concentration of 0.5 mM. |
| Targets | COX | MAPK | MEK | PPAR | FAS | ERK |
| In vitro | 2,4,5-trimethoxybenzaldehyde, a bitter principle in plants, suppresses adipogenesis through the regulation of ERK1.[Pubmed: 25222709 ]J Agric Food Chem. 2014 Oct 8;62(40):9860-7.We demonstrated that 2,4,5-Trimethoxybenzaldehyde (2,4,5-TMBA), a bitter principle in plants and a natural cyclooxygenase II (COX-2) inhibitor, suppressed the differentiation of preadipocyts into adipocytes at the concentration of 0.5 mM.
Production of a COX-2 inhibitor, 2,4,5-trimethoxybenzaldehyde, with submerged cultured Antrodia camphorata.[Pubmed: 17397476]Lett Appl Microbiol. 2007 Apr;44(4):387-92.To investigate the active ingredient in fruiting bodies and to produce it with cultured mycelium in Antrodia camphorata (BCRC 35398).
|

2,4,5-Trimethoxybenzaldehyde Dilution Calculator

2,4,5-Trimethoxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0968 mL | 25.4842 mL | 50.9684 mL | 101.9368 mL | 127.421 mL |
| 5 mM | 1.0194 mL | 5.0968 mL | 10.1937 mL | 20.3874 mL | 25.4842 mL |
| 10 mM | 0.5097 mL | 2.5484 mL | 5.0968 mL | 10.1937 mL | 12.7421 mL |
| 50 mM | 0.1019 mL | 0.5097 mL | 1.0194 mL | 2.0387 mL | 2.5484 mL |
| 100 mM | 0.051 mL | 0.2548 mL | 0.5097 mL | 1.0194 mL | 1.2742 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Asaraldehyde is a natural COX-2 inhibitor, exhibiting 17-fold selectivity over COX-1.
- Azathioprine
Catalog No.:BCC4762
CAS No.:446-86-6
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Homoeriodictyol
Catalog No.:BCN6804
CAS No.:446-71-9
- BMS CCR2 22
Catalog No.:BCC7572
CAS No.:445479-97-0
- SGS 518 oxalate
Catalog No.:BCC7750
CAS No.:445441-27-0
- BMS-345541(free base)
Catalog No.:BCC5374
CAS No.:445430-58-0
- ZM 449829
Catalog No.:BCC2444
CAS No.:4452-06-6
- 1,4:3,6-Dianhydro-α-D-glucopyranose
Catalog No.:BCC8420
CAS No.:4451-30-3
- EBPC
Catalog No.:BCC6677
CAS No.:4450-98-0
- TC-O 9311
Catalog No.:BCC7900
CAS No.:444932-31-4
- AM1241
Catalog No.:BCC2551
CAS No.:444912-48-5
- Warangalone
Catalog No.:BCN4788
CAS No.:4449-55-2
- YM 230888
Catalog No.:BCC5956
CAS No.:446257-23-4
- 4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one
Catalog No.:BCC8646
CAS No.:446292-10-0
- RepSox
Catalog No.:BCC1887
CAS No.:446859-33-2
- Angiotensin II human
Catalog No.:BCC4087
CAS No.:4474-91-3
- RLLFT-NH2
Catalog No.:BCC3954
CAS No.:447408-68-6
- Ruixianglangdusu B
Catalog No.:BCN6869
CAS No.:447454-49-1
- Sulforaphane
Catalog No.:BCN2349
CAS No.:4478-93-7
- Betulonic acid
Catalog No.:BCN5500
CAS No.:4481-62-3
- NS 1643
Catalog No.:BCC7552
CAS No.:448895-37-2
- WY 45233 succinate
Catalog No.:BCC6125
CAS No.:448904-47-0
- 3-(4-Methoxyphenyl)-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN1440
CAS No.:448905-82-6
- ITE
Catalog No.:BCC3902
CAS No.:448906-42-1
[Chemical constituents from rhizomes of Acorus tatarinowii].[Pubmed:23713285]
Zhongguo Zhong Yao Za Zhi. 2013 Feb;38(4):569-73.
Fifteen compounds were isolated from the rhizomes of Acorus tatarinowii by means of various chromatographic techniques such as silica gel, ODS, Sephadex LH-20 and preparative HPLC, and their structures were elucidated as tatanone A (1), calamusenone (2), acoronene (3), 2-acetyloxyacoronene (4), acorenone (5), alpha-asarone (6), beta-asarone (7), 1,2-dimethoxy-4-(1'Z-propenyl) benzene (8), methyleugenol (9), asarylaldehyde (10), acoramone (11), gamma-asarone (12), 5-hydroxymethyl-2-furaldehyde (13), galgravin (14) and eudesmin (15) on the basis of spectroscopic data analysis. Compound 1 was a new compound, and compounds 3-5 were separated from Acorus species for the first time.
Production of a COX-2 inhibitor, 2,4,5-trimethoxybenzaldehyde, with submerged cultured Antrodia camphorata.[Pubmed:17397476]
Lett Appl Microbiol. 2007 Apr;44(4):387-92.
AIMS: To investigate the active ingredient in fruiting bodies and to produce it with cultured mycelium in Antrodia camphorata (BCRC 35398). METHODS AND RESULTS: The volatile components from the fruiting bodies, the liquid cultured broth of A. camphorata and Cinnamomum kanehirae wood were separately isolated by steam distillation-solvent extraction and identified by gas chromatography-mass spectrometry. In the fruiting bodies, a COX-2 inhibitor 2,4,5-Trimethoxybenzaldehyde (TMBA) was found to be the most abundant constituent, but was totally absent in its cultured broth and its natural host, C. kanehirae wood. On feeding with the acid-digested sawdust of C. kanehirae wood or vanillin to the broth for culture, TMBA was produced in both cultured broths. CONCLUSION: The TMBA identified in fruiting bodies was an active ingredient whose functions consisted with the reported experiences of this mushroom. Feeding vanillin to culture broth could produce TMBA containing mycelium product like its fruiting bodies did. SIGNIFICANCE AND IMPACT OF THE STUDY: This study found an active ingredient in fruiting bodies of A. camphorata and elucidated this compound derived from digested sawdust of C. kanehirae wood. A feasible method was also developed to produce TMBA containing mycelium by feeding vanillin.
2,4,5-trimethoxybenzaldehyde, a bitter principle in plants, suppresses adipogenesis through the regulation of ERK1.[Pubmed:25222709]
J Agric Food Chem. 2014 Oct 8;62(40):9860-7.
Because of the prevalence of obesity, there is particular interest in finding potential therapeutic targets. In a previous study, we demonstrated that 2,4,5-Trimethoxybenzaldehyde (2,4,5-TMBA), a bitter principle in plants and a natural cyclooxygenase II (COX-2) inhibitor, suppressed the differentiation of preadipocyts into adipocytes at the concentration of 0.5 mM. In this current study, we aimed to investigate the stage during adipogenesis that is critically affected by 2,4,5-TMBA and the effects of 2,4,5-TMBA on the time-course expression of signaling molecules MAP kinase kinase (MAPKK, represented by MEK) and extracellular signal-regulated kinase (ERK), transcription factors CCAAT/enhancer binding protein (C/EBP)alpha, beta, and delta and peroxisome proliferator-activated receptor (PPAR)gamma, lipogenic enzymes acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), and lipid droplet-coating protein perilipin A. When preadipocytes were co-cultured with 2,4,5-TMBA (0.5 mM) specifically at post-induction days 0-2, 2-4, 4-6, or 6-8 only, relative lipid accumulation was decreased by 67.93, 34.65, 49.56, and 34.32%, respectively. A time-course study showed that treatment of 2,4,5-TMBA suppressed the phosphorylation of ERK1 at the initial stage of adipogenesis but upregulated the phosphorylation at the late stage, which is opposite to the conditions required for the differentiation process. The overall expression of C/EBPalpha, beta, and delta, PPARgamma2, ACC, FAS, and perilipin A in preadipocytes was downregulated by the treatment of 2,4,5-TMBA. Taken together, our findings suggest that 2,4,5-TMBA suppresses adipogenesis through the regulation of ERK1 phosphorylation. Although results from in vitro studies cannot be directly extrapolated into clinical effects, our study will help to elucidate the anti-adipogenic potential of 2,4,5-TMBA.


