17-DMAG (Alvespimycin) HClHsp90 inhibitor CAS# 467214-21-7 |
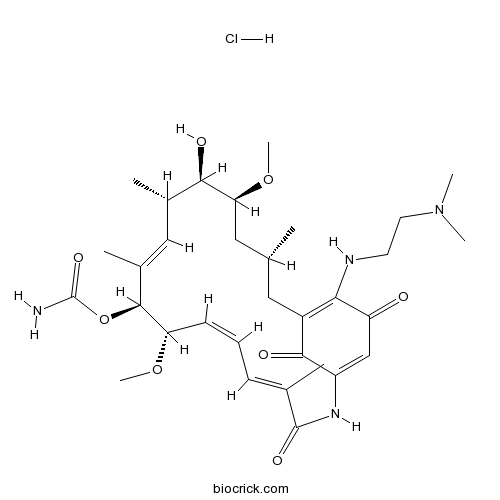
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 467214-21-7 | SDF | Download SDF |
| PubChem ID | 9852573 | Appearance | Powder |
| Formula | C32H49ClN4O8 | M.Wt | 653.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 707545 | ||
| Solubility | DMSO : ≥ 50 mg/mL (76.55 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-21-[2-(dimethylamino)ethylamino]-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate;hydrochloride | ||
| SMILES | CC1CC(C(C(C=C(C(C(C=CC=C(C(=O)NC2=CC(=O)C(=C(C1)C2=O)NCCN(C)C)C)OC)OC(=O)N)C)C)O)OC.Cl | ||
| Standard InChIKey | DFSYBWLNYPEFJK-IHLRWNDRSA-N | ||
| Standard InChI | InChI=1S/C32H48N4O8.ClH/c1-18-14-22-27(34-12-13-36(5)6)24(37)17-23(29(22)39)35-31(40)19(2)10-9-11-25(42-7)30(44-32(33)41)21(4)16-20(3)28(38)26(15-18)43-8;/h9-11,16-18,20,25-26,28,30,34,38H,12-15H2,1-8H3,(H2,33,41)(H,35,40);1H/b11-9-,19-10+,21-16+;/t18-,20+,25+,26+,28-,30+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Water-soluble analog of 17-AAG and geldanamycin. Binds the ATP binding site of Hsp90 and inhibits its chaperone activity. Displays more potent antitumor activity than 17-AAG (mean GI50 values are 53 and 123 nM for 17-DMAG and 17-AAG respectively). |

17-DMAG (Alvespimycin) HCl Dilution Calculator

17-DMAG (Alvespimycin) HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5309 mL | 7.6545 mL | 15.309 mL | 30.618 mL | 38.2725 mL |
| 5 mM | 0.3062 mL | 1.5309 mL | 3.0618 mL | 6.1236 mL | 7.6545 mL |
| 10 mM | 0.1531 mL | 0.7655 mL | 1.5309 mL | 3.0618 mL | 3.8273 mL |
| 50 mM | 0.0306 mL | 0.1531 mL | 0.3062 mL | 0.6124 mL | 0.7655 mL |
| 100 mM | 0.0153 mL | 0.0765 mL | 0.1531 mL | 0.3062 mL | 0.3827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
17-DMAG is an inhibitor of Hsp90 with IC50 value of 62±29nM [1].
17-DMAG can bind to the ATP-binding motif of Hsp90 and inhibit the protein chaperoning activity of Hsp90. It will cause misfolding and subsequent degradation of Hsp90’s client proteins, such as EGFR, AKT, mutant p53, and IKK. Since there are more speci?c conformation Hsp90 required for 17-DMAG binding in tumor cells and many client proteins of Hsp90 contribute to tumor cell growth, 17-DMAG is usually more toxic to tumor cells than to normal cells [2].
17-DMAG is reported as an antitumor agent with more broadly exploitable activity and more pharmaceutically tractable characteristics in the in vitro and initial in vivo assay. 17-DMAG can effect cell growth when treating the NCI 60 cell lines with it, the mean GI50 is 0.053mM. The in vivo activity of 17-DMAG is tested in four melanoma models using the Freiburg human tumor xenograft panel and two lung xenografts. It shows that 17-DMAG has high activity in the two lung xenografts and two of the four melanoma models, but not in another two, MEXF 462 and MEXF 514 [3].
References:
[1] Jie Ge, Emmanuel Normant, James R. Porter, Janid A. Ali, Marlene S. Dembski, Yun Gao, Asimina T. Georges, Louis Grenier, Roger H. Pak, Jon Patterson, Jens R. Sydor, Thomas T. Tibbitts, Jeffrey K. Tong, Julian Adams, and Vito J. Palombella. Design, synthesis and biological evaluation of Hydroquinone derivatives of 17-Amino-17-demethoxygeldanamycin as potent, water-soluble inhibitors of Hsp90. J. Med. Chem. 2006, 49, 4606-4615.
[2] Xiaoping Sun, Jillian A. Bristol, Satoko Iwahori, Stacy R. Hagemeier, Qiao Meng, Elizabeth A. Barlow, Joyce D. Fingeroth, Vera L. Tarakanova, Robert F. Kalejta, Shannon C. Kenney. Hsp90 Inhibitor 17-DMAG Decreases Expression of Conserved Herpesvirus Protein Kinases and Reduces Virus Production in
Epstein-Barr Virus-Infected Cells. Journal of Virology. 2013, 87 (18): 10126–10138.
[3] Melinda Hollingshead, Michael Alley, Angelika M. Burger, Suzanne Borgel,Christine Pacula-Cox, Heinz-Herbert Fiebig, Edward A. Sausville. In vivo antitumor ef?cacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol. 2005, 56: 115–125.
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- Theaflavin
Catalog No.:BCN5419
CAS No.:4670-05-7
- Rehmannic acid
Catalog No.:BCN4632
CAS No.:467-81-2
- Coronaridine
Catalog No.:BCN3762
CAS No.:467-77-6
- Hecogenin
Catalog No.:BCN5408
CAS No.:467-55-0
- N-Methylcorydiniumiodide
Catalog No.:BCN7873
CAS No.:4668-64-6
- Z-Asp-OMe
Catalog No.:BCC2792
CAS No.:4668-42-2
- 3-O-(2'E ,4'E-decadienoyl)-20-O-acetylingenol
Catalog No.:BCN1437
CAS No.:466663-12-7
- 3-O-(2'E,4'E-Decadienoyl)ingenol
Catalog No.:BCN3768
CAS No.:466663-11-6
- Arteminin
Catalog No.:BCN3642
CAS No.:466639-11-2
- Cryptomeridiol
Catalog No.:BCN5516
CAS No.:4666-84-6
- Rauvomitin
Catalog No.:BCN3421
CAS No.:466-57-9
- Diphenyleneiodonium chloride
Catalog No.:BCC6670
CAS No.:4673-26-1
- Nootkatone
Catalog No.:BCN5517
CAS No.:4674-50-4
- Lu AE58054 Hydrochloride
Catalog No.:BCC1708
CAS No.:467458-02-2
- Lu AE58054
Catalog No.:BCC1707
CAS No.:467459-31-0
- Colupulone
Catalog No.:BCN8097
CAS No.:468-27-9
- Lupulon
Catalog No.:BCC8204
CAS No.:468-28-0
- Mesembrenone
Catalog No.:BCN3753
CAS No.:468-54-2
- Drimenol
Catalog No.:BCN7224
CAS No.:468-68-8
- Orphenadrine Citrate
Catalog No.:BCC4572
CAS No.:4682-36-4
- Norscopolamine
Catalog No.:BCN3983
CAS No.:4684-28-0
- Picrinine
Catalog No.:BCN5518
CAS No.:4684-32-6
- Dihydrocorynantheine
Catalog No.:BCN3747
CAS No.:4684-43-9
Inhibition of hsp90 compromises the DNA damage response to radiation.[Pubmed:16982765]
Cancer Res. 2006 Sep 15;66(18):9211-20.
Inhibitors of the molecular chaperone Hsp90 have been shown to enhance tumor cell radiosensitivity. To begin to address the mechanism responsible, we have determined the effect of the Hsp90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17DMAG) on the DNA damage response to radiation. Exposure of MiaPaCa tumor cells to 17DMAG, which results in radiosensitization, inhibited the repair of DNA double-strand breaks according to gammaH2AX foci dispersal and the neutral comet assay. This repair inhibition was associated with reduced DNA-PK catalytic subunit (DNA-PKcs) phosphorylation after irradiation and a disruption of DNA-PKcs/ErbB1 interaction. These data suggest that the previously established 17DMAG-mediated reduction in ErbB1 activity reduces its interaction with DNA-PKcs and thus accounts for the attenuation of radiation-induced DNA-PK activation. 17DMAG was also found to abrogate the activation of the G(2)- and S-phase cell cycle checkpoints. Associated with these events was a reduction in radiation-induced ataxia-telangiectasia mutated (ATM) activation and foci formation in 17DMAG-treated cells. Although no interaction between ATM and Hsp90 was detected, Hsp90 was found to interact with the MRE11/Rad50/NBS1 (MRN) complex. 17DMAG exposure reduced the ability of the MRN components to form nuclear foci after irradiation. Moreover, 17DMAG exposure reduced the interaction between NBS1 and ATM, although no degradation of the MRN complex was detected. These results suggest that the diminished radiation-induced activation of ATM in 17DMAG-treated cells was the result of a compromise in the function of the MRN complex. These data indicate that Hsp90 can contribute to the DNA damage response to radiation affecting both DNA repair and cell cycle checkpoint activation.
Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models.[Pubmed:15841378]
Cancer Chemother Pharmacol. 2005 Aug;56(2):126-37.
The heat shock protein Hsp90 is a potential target for drug discovery of novel anticancer agents. By affecting this protein, several cell signaling pathways may be simultaneously modulated. The geldanamycin analog 17AAG has been shown to inhibit Hsp90 and associated proteins. Its clinical use, however, is hampered by poor solubility and thus, difficulties in formulation. Therefore, a water-soluble derivative was desirable and 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) is such a derivative. Studies were carried out in order to evaluate the activity and molecular mechanism(s) of 17DMAG in comparison with those of 17-allylamino-demethoxygeldanamycin (17AAG). 17DMAG was found to be more potent than 17AAG in a panel of 64 different patient-derived tumor explants studied in vitro in the clonogenic assay. The tumor types that responded best included mammary cancers (six of eight), head and neck cancers (two of two), sarcomas (four of four), pancreas carcinoma (two of three), colon tumors (four of eight for 17AAG and six of eight for 17DMAG), and melanoma (two of seven). Bioinformatic comparisons suggested that, while 17AAG and 17DMAG are likely to share the same mode(s) of action, there was very little similarity with standard anticancer agents. Using three permanent human melanoma cell lines with differing sensitivities to 17AAG and 17DMAG (MEXF 276L, MEXF 462NL and MEXF 514L), we found that Hsp90 protein was reduced following treatment at a concentration associated with total growth inhibition. The latter occurred in MEXF 276L cells only, which are most sensitive to both compounds. The depletion of Hsp90 was more pronounced in cells exposed to 17DMAG than in those treated with 17AAG. The reduction in Hsp90 was associated with the expression of erbB2 and erbB3 in MEXF 276L, while erbB2 and erbB3 were absent in the more resistant MEXF 462NL and MEXF 514L cells. Levels of known Hsp90 client proteins such as phosphorylated AKT followed by AKT, cyclin D1 preceding cdk4, and craf-1 declined as a result of drug treatment in all three melanoma cell lines. However, the duration of drug exposure needed to achieve these effects was variable. All cell lines showed increased expression of Hsp70 and activated cleavage of PARP. No change in PI3K expression was observed and all melanoma cells were found to harbor the activating V599E BRAF kinase mutation. The results of our in vitro studies are consistent with both 17AAG and 17DMAG acting via the same molecular mechanism, i.e. by modulating Hsp90 function. Since 17DMAG can be formulated in physiological aqueous solutions, the data reported here strongly support the development of 17DMAG as a more pharmaceutically practicable congener of 17AAG.
Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats.[Pubmed:11855755]
Cancer Chemother Pharmacol. 2002 Jan;49(1):7-19.
PURPOSE: 17-(Dimethylaminoethylamino)-17-demethoxygeldanamycin (17DMAG) is an analogue of the benzoquinone ansamycin compound 17-(allylamino)-17-demethoxygeldanamycin (17AAG), which is currently being evaluated in clinical trials. Studies were performed in mice and rats to: (1) define the plasma pharmacokinetics, tissue distribution, and urinary excretion of 17DMAG after i.v. delivery; (2) define the bioavailability of 17DMAG after i.p. and oral delivery; (3) characterize the biliary excretion of 17DMAG after i.v. delivery to rats; and (4) characterize, if possible, any metabolites of 17DMAG observed in plasma, tissue, urine, or bile. MATERIALS AND METHODS: Studies were performed in female, CD2F1 mice or male Fischer 344 rats. In preliminary toxicity studies and subsequent i.v. pharmacokinetic studies in mice, 17DMAG i.v. bolus doses of 33.3, 50, and 75 mg/kg were used. In bioavailability studies, i.p. and oral 17DMAG doses of 75 mg/kg were used. In preliminary toxicity studies in rats, i.v. bolus doses of 10 and 20 mg/kg were used, and in i.v. pharmacokinetic studies 10 mg/kg was used. Compartmental and noncompartmental analyses were applied to the plasma concentration versus time data. In mice and rats, concentrations of 17DMAG were determined in multiple tissues. Urine was collected from mice and rats treated with each of the i.v. doses of 17DMAG mentioned above, and drug excretion was calculated until 24 h after treatment. Biliary excretion of 17DMAG and metabolites was studied in bile duct-cannulated rats given a 10 mg/kg i.v. bolus dose of 17DMAG. 17DMAG metabolites were identified with LC/MS. RESULTS: A 75 mg/kg dose of 17DMAG caused no changes in appearance, appetite, waste elimination, or survival of treated mice as compared to vehicle-treated controls. Bolus i.v. delivery of 17DMAG at 75 mg/kg produced "peak" plasma 17DMAG concentrations between 18 and 24.2 microg/ml in mice killed at 5 min after injection. Sequential reduction in the 17DMAG dose to 50 and 33.3 mg/kg resulted in "peak" plasma 17DMAG concentrations between 9.4 and 14.4, and 8.4 and 10.5 microg/ml, respectively. Plasma 17DMAG AUC increased from 362 to 674 and 1150 microg/ml x min when the 17DMAG dose increased from 33.3 to 50 and 75 mg/kg, respectively, corresponding to a decrease in 17DMAG CLtb from 92 ml/min per kg to 75 and 65 ml/min per kg. Plasma 17DMAG concentration versus time data were best fit by a two-compartment open linear model. No potential 17DMAG metabolites were observed in plasma. 17DMAG bioavailability was 100% and 50% after i.p. and oral delivery, respectively. In rats, an i.v. bolus dose of 10 mg/kg produced peak plasma 17DMAG concentrations between 0.88 and 1.74 microg/ml. Plasma 17DMAG concentrations had fallen below the lower limit of quantitation by 180 min and were best fit by a one-compartment open linear model. The plasma 17DMAG AUC was 104 microg/ml x min, corresponding to a 17DMAG CLtb of 96 ml/min per kg. 17DMAG distributed rapidly to all mouse and rat tissues except brain and testes. Only mouse liver contained materials consistent with potential metabolites of 17DMAG, but their concentrations were below the limit of quantitation of the HPLC assay used. Within the first 24 h after delivery, urinary excretion of 17DMAG by mice and rats accounted for 10.6-14.8% and 12.5-16%, respectively, of the delivered dose. By 15 min after i.v. delivery of 10 mg/kg of 17DMAG, rat bile contained 11 new materials with absorbance similar to that of 17DMAG. Four of these proposed metabolites had an Mr of 633, indicating addition of an oxygen. Two of these proposed metabolites had an Mr of 603, implying the loss of one methyl group, and one had an Mr of 589, implying the loss of two methyl groups. The remaining four proposed metabolites had an Mr of 566, 571, 629, and 645, respectively. Biliary excretion of 17DMAG and metabolites accounted for 4.7 +/- 1.4% of the delivered dose, with 17DMAG accounting for 50.7 +/- 3.4% of the biliary excretion. CONCLUSIONS: 17DMAG has excellent bioavailability when given i.p. and good bioavailability when given orally. 17DMAG is widely distributed to tissues and is quantitatively metabolized much less than is 17AAG. The pharmacokinetic and metabolite data generated should prove relevant to the design of additional preclinical studies as well as to contemplated clinical trials of 17DMAG and could be useful in their interpretation.


