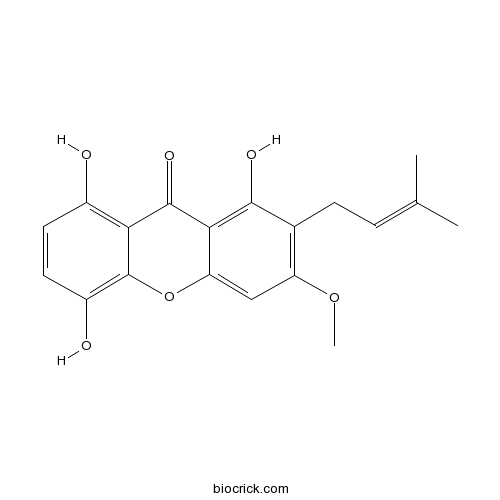
1,5,8-Trihydroxy-3-methoxy-2-prenylxanthoneCAS# 110187-11-6 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 110187-11-6 | SDF | Download SDF |
| PubChem ID | 14162674 | Appearance | Yellow powder |
| Formula | C19H18O6 | M.Wt | 342.4 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,5,8-trihydroxy-3-methoxy-2-(3-methylbut-2-enyl)xanthen-9-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1O)C(=O)C3=C(C=CC(=C3O2)O)O)OC)C | ||
| Standard InChIKey | AAANZTDKTFGJLZ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone Dilution Calculator

1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9206 mL | 14.6028 mL | 29.2056 mL | 58.4112 mL | 73.014 mL |
| 5 mM | 0.5841 mL | 2.9206 mL | 5.8411 mL | 11.6822 mL | 14.6028 mL |
| 10 mM | 0.2921 mL | 1.4603 mL | 2.9206 mL | 5.8411 mL | 7.3014 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5841 mL | 1.1682 mL | 1.4603 mL |
| 100 mM | 0.0292 mL | 0.146 mL | 0.2921 mL | 0.5841 mL | 0.7301 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- JZL184
Catalog No.:BCC4790
CAS No.:1101854-58-3
- Ouabain Octahydrate
Catalog No.:BCC5211
CAS No.:11018-89-6
- 4-Galloylquinic acid
Catalog No.:BCN3733
CAS No.:110170-37-1
- Methyl hesperidin
Catalog No.:BCN6341
CAS No.:11013-97-1
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Malonylginsenoside Rb(1)
Catalog No.:BCC9230
CAS No.:88140-34-5
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
- Temocapril HCl
Catalog No.:BCC5016
CAS No.:110221-44-8
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Amrubicin
Catalog No.:BCC3640
CAS No.:110267-81-7
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
No significant difference between chiari malformation type 1.5 and type I.[Pubmed:28384597]
Clin Neurol Neurosurg. 2017 Jun;157:34-39.
OBJECTIVE: Chiari malformation Type 1.5 (CM 1.5) was defined as the association of Chiari malformation Type I (CM I) and brainstem herniation. The objective was to demonstrate the difference of clinical features and surgical outcomes between CM 1.5 and CM I. PATIENTS AND METHODS: All CM 1.5 and CM I adult patients who underwent posterior fossa decompression with duraplasty at our institution between 2006 and 2010 were retrospectively reviewed. Clinical characteristics, imaging features, and long-term outcomes were compared between CM 1.5 and CM I patients. RESULTS: A total of 142 adult patients were enrolled, including 27 CM 1.5 and 115 CM I patients. The average follow-up period was 102 months. Age at diagnosis was significantly younger in CM 1.5 group than CM I group (p=0.039). And the degree of tonsillar herniation was significantly more severe in CM 1.5 group than CM I group (p<0.001). There was no significant difference in other clinical and imaging characteristics. Moreover, improvement of symptoms was observed in 21 CM 1.5 patients (77.8%) and 94 CM I patients (81.7%), and no significant difference was detected (p=0.637). There was no significant difference in the resolution of syringomyelia between CM 1.5 (72.7%) and CM I (76.5%) patients, either (p=0. 710). CONCLUSIONS: Although CM 1.5 patients presented with brainstem herniation and more severe tonsillar herniation, other clinical and imaging features and surgical outcomes were similar with CM I patients. We think CM 1.5 is just a subtype of CM I, rather than a unique type of Chiari malformations.
53 W average power CEP-stabilized OPCPA system delivering 5.5 TW few cycle pulses at 1 kHz repetition rate.[Pubmed:28380838]
Opt Express. 2017 Mar 6;25(5):5797-5806.
We present a high peak and average power optical parametric chirped pulse amplification system driven by diode-pumped Yb:KGW and Nd:YAG lasers running at 1 kHz repetition rate. The advanced architecture of the system allows us to achieve >53 W average power combined with 5.5 TW peak power, along with sub-220 mrad CEP stability and sub-9 fs pulse duration at a center wavelength around 880 nm. Broadband, background-free, passively CEP stabilized seed pulses are produced in a series of cascaded optical parametric amplifiers pumped by the Yb:KGW laser, while a diode-pumped Nd:YAG laser system provides multi-mJ pump pulses for power amplification stages. Excellent stability of output parameters over 16 hours of continuous operation is demonstrated.
Atomic frequency reference at 1033 nm for ytterbium (Yb)-doped fiber lasers and applications exploiting a rubidium (Rb) 5S1/2 to 4D5/2 one-colour two-photon transition.[Pubmed:28380912]
Opt Express. 2017 Apr 3;25(7):7960-7969.
We demonstrate a two-photon transition of rubidium (Rb) atoms from the ground state (5S1/2) to the excited state (4D5/2), using a home-built ytterbium (Yb)-doped fiber amplifier at 1033 nm. This is the first demonstration of an atomic frequency reference at 1033 nm as well as of a one-colour two-photon transition for the above energy levels. A simple optical setup is presented for the two-photon transition fluorescence spectroscopy, which is useful for frequency stabilization for a broad class of lasers. This spectroscopy has potential applications in the fiber laser industry as a frequency reference, particularly for the Yb-doped fiber lasers. This two-photon transition also has applications in atomic physics as a background- free high- resolution atom detection and for quantum communication, which is outlined in this article.
A Novel Methodology for Synthesis of 1,5,6-Trisubstituted 2(1H)-Pyrazinones of Biological Interest.[Pubmed:28381677]
Chem Pharm Bull (Tokyo). 2017;65(4):365-372.
In this report, we describe a new method for the synthesis of densely functionalized 2(1H)-pyrazinones. Treatment of mesoionic 1,3-oxazolium-5-olates with carbanions derived from activated methylene isocyanides (p-toluenesulfonylmethyl isocyanide (TosMIC) and ethyl isocyanoacetate) causes a novel ring transformation affording 2(1H)-pyrazinones in moderate to high yields. The cytotoxicity and antibacterial activity of some of the obtained products were studied and some of the products exhibited tumor-specific cytotoxicity.


