1,2,3,6-TetragalloylglucoseCAS# 79886-50-3 |
Quality Control & MSDS
Number of papers citing our products

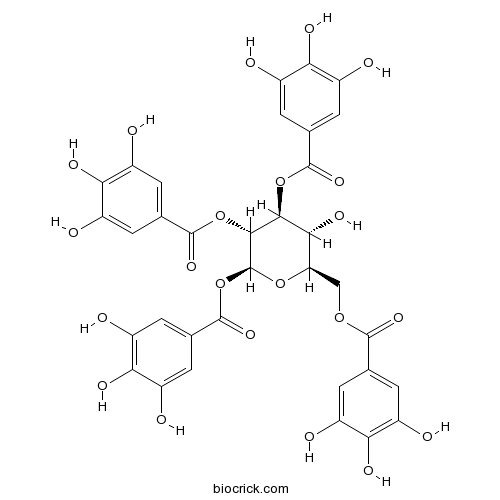
Chemical structure

3D structure
| Cas No. | 79886-50-3 | SDF | Download SDF |
| PubChem ID | 73178 | Appearance | Powder |
| Formula | C34H28O22 | M.Wt | 788.57 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3R,4S,5R,6S)-3-hydroxy-4,5,6-tris[(3,4,5-trihydroxybenzoyl)oxy]oxan-2-yl]methyl 3,4,5-trihydroxybenzoate | ||
| SMILES | C1=C(C=C(C(=C1O)O)O)C(=O)OCC2C(C(C(C(O2)OC(=O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C(=C5)O)O)O)O | ||
| Standard InChIKey | RATQVALKDAUZBW-XPMKZLBQSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 1,2,3,6-Tetragalloylglucose has antioxidative activity. 2. 1,2,3,6-Tetragalloylglucose shows the most potent anticomplement activity (IC(50), 34 microM). |
| Targets | LDL |

1,2,3,6-Tetragalloylglucose Dilution Calculator

1,2,3,6-Tetragalloylglucose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2681 mL | 6.3406 mL | 12.6812 mL | 25.3624 mL | 31.703 mL |
| 5 mM | 0.2536 mL | 1.2681 mL | 2.5362 mL | 5.0725 mL | 6.3406 mL |
| 10 mM | 0.1268 mL | 0.6341 mL | 1.2681 mL | 2.5362 mL | 3.1703 mL |
| 50 mM | 0.0254 mL | 0.1268 mL | 0.2536 mL | 0.5072 mL | 0.6341 mL |
| 100 mM | 0.0127 mL | 0.0634 mL | 0.1268 mL | 0.2536 mL | 0.317 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Castanospermine
Catalog No.:BCC6783
CAS No.:79831-76-8
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- H-Hyp(tBu)-OH
Catalog No.:BCC3249
CAS No.:79775-07-8
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
- Linifanib (ABT-869)
Catalog No.:BCC1261
CAS No.:796967-16-3
- Crassicauline A
Catalog No.:BCN2516
CAS No.:79592-91-9
- Alarelin Acetate
Catalog No.:BCC1336
CAS No.:79561-22-1
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
- 1,7-Diphenyl-4-hepten-3-one
Catalog No.:BCN3592
CAS No.:79559-59-4
- 5-Ethoxychelerthrine
Catalog No.:BCC8105
CAS No.:79559-55-0
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
- Simvastatin
Catalog No.:BCN2569
CAS No.:79902-63-9
- Forsythoside A
Catalog No.:BCN1195
CAS No.:79916-77-1
- ML130 (Nodinitib-1)
Catalog No.:BCC4611
CAS No.:799264-47-4
- Idazoxan hydrochloride
Catalog No.:BCC6798
CAS No.:79944-56-2
- Boc-His(Bom)-OH
Catalog No.:BCC3400
CAS No.:79950-65-5
- Quinovic acid 3-O-beta-D-glucoside
Catalog No.:BCN4334
CAS No.:79955-41-2
- Fmoc-D-Ala-OH
Catalog No.:BCC3036
CAS No.:79990-15-1
- Blumeatin B
Catalog No.:BCN4335
CAS No.:79995-67-8
- Dapsone
Catalog No.:BCC5220
CAS No.:80-08-0
- Sulfamethoxypyridazine
Catalog No.:BCC4728
CAS No.:80-35-3
- Homatropine Methylbromide
Catalog No.:BCC4571
CAS No.:80-49-9
- Alpha-pinene
Catalog No.:BCN3855
CAS No.:80-56-8
Anti-complement activity of constituents from the stem-bark of Juglans mandshurica.[Pubmed:12843637]
Biol Pharm Bull. 2003 Jul;26(7):1042-4.
Four known flavonoids and two galloyl glucoses isolated from the stem-bark of Juglans mandshurica (Juglandaceae), namely taxifolin (1), afzelin (2), quercitrin (3), myricitrin (4), 1,2,6-trigalloylglucose (5), and 1,2,3,6-Tetragalloylglucose (6), were evaluated for their anti-complement activity against complement system. Afzelin (2) and quercitrin (3) showed inhibitory activity against complement system with 50% inhibitory concentrations (IC(50)) values of 258 and 440 microM. 1,2,6-Trigalloylglucose (5) and 1,2,3,6-Tetragalloylglucose (6) exhibited anti-complement activity with IC(50) values of 136 and 34 microM. In terms of the evaluation of the structure-activity relationship of 3,5,7-trihydroxyflavone, compounds 2, 3, and 4 were hydrolyzed with naringinase to give kaempferol (2a), quercetin (3a), and myricetin (4a) as their aglycones, and these were also tested for their anti-complement activity. Of the three aglycones, kaempferol (2a) exhibited weak anti-complement activity with an IC(50) value of 730 microM, while quercetin (3a) and myricetin (4a) were inactive in this assay system. Among the compounds tested, 1,2,3,6-Tetragalloylglucose (6) showed the most potent anticomplement activity (IC(50), 34 microM).
Inhibition effects of the classical pathway complement of isolated compounds from Quercus glauca.[Pubmed:21078772]
Hum Exp Toxicol. 2011 Sep;30(9):1415-9.
Species of the Quercus species is an evergreen broadleaf tree found not only in Korea but also in China, Taiwan, and Japan. Quercus species is the most commonly occurring plant among the 50 native species of the family Fagaceae in Korea, China, and Taiwan. Quercus species have been used for diarrhea, dysentery, dermatitis, and hemorrhagia in Korean folk medicine. The present study evaluated the anticomplement effect of constituents from Quercus species (Fagaceae) in classical pathway complement system. We have evaluated leaves of five species of the Quercus genus with regard to its anticomplement activity and have identified its active principles following activity-guided isolation. Bioactivity-guided fractionation of the 80% methanol extracts of the stem barks of Quercus glauca Thunberg has led to the isolation of galloyl derivatives, displaying high anticomplement activity. Four galloyl derivatives isolated from the leaves of Q. glauca, namely 6'-O-galloyl salidroside (1), methyl gallate (2), 1,2,3,6-Tetragalloylglucose (3), and 1,2,6-trigalloylglucose (4). 1, 2, 3 and 4 showed inhibitory activity against complement system with 50% inhibitory concentrations (IC(50)) values of 224 muM, 362.4 muM, 32.3 muM, and 138.3 muM. Among the compounds tested, 3 showed the most potent anticomplement activity (IC(50), 32.3 muM). This is the first report of the isolation and anticomplement activity from Q. glauca.
Antioxidative activities of galloyl glucopyranosides from the stem-bark of Juglans mandshurica.[Pubmed:18685223]
Biosci Biotechnol Biochem. 2008 Aug;72(8):2158-63.
Two phenolics, 1,2,6-trigalloylglucose (1) and 1,2,3,6-Tetragalloylglucose (2), isolated from the stem-bark of Juglans mandshurica were evaluated for their antioxidative activities. The results showed that compounds 1 and 2 exhibited strong scavenging activities against 1,1'-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis-(3-ethylbenzenthiazoline-6-sulphonic) acid (ABTS(*+)), and superoxide radicals (O(2)(*-)), and also had a significant inhibitory effect on lipid peroxidation and low-density lipoprotein (LDL) oxidation. The strong superoxide radical scavenging of 1 and 2 resulted from the potential competitive inhibition with xanthine at the active site of xanthine oxidase (OX). In addition, compounds 1 and 2 displayed significant lipoxygenase inhibitory activity, the mode of inhibition also being identified as competitive. In comparison, the antioxidative activities of compounds 1 and 2, together with gallic acid, indicated that the number of galloyl moieties could play an important role in the antioxidative activity.


