(d(CH2)51,Tyr(Me)2,Arg8)-VasopressinSelective vasopressin V1A antagonist CAS# 73168-24-8 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 73168-24-8 | SDF | Download SDF |
| PubChem ID | 9833460 | Appearance | Powder |
| Formula | C52H74N14O12S2 | M.Wt | 1151.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
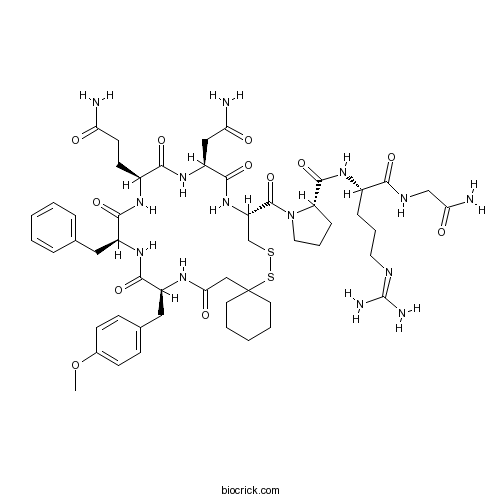
| Sequence | YFQNCPRG (Modifications: Tyr-1 = Pmp-Tyr(Me), Gly-8 = C-terminal amide) | ||
| Chemical Name | (2S)-N-[(2S)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]-1-[(10R,13S,16S,19S,22S)-13-(2-amino-2-oxoethyl)-16-(3-amino-3-oxopropyl)-19-benzyl-22-[(4-methoxyphenyl)methyl]-12,15,18,21,24-pentaoxo-7,8-dithia-11,14,17,20,23-pentazaspiro[5.19]pentacosane-10-carbonyl]pyrrolidine-2-carboxamide | ||
| SMILES | COC1=CC=C(C=C1)CC2C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(CSSC3(CCCCC3)CC(=O)N2)C(=O)N4CCCC4C(=O)NC(CCCN=C(N)N)C(=O)NCC(=O)N)CC(=O)N)CCC(=O)N)CC5=CC=CC=C5 | ||
| Standard InChIKey | QVQOGNOOAMQKCE-ZTYVOHGWSA-N | ||
| Standard InChI | InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58)/t33-,34-,35-,36-,37-,38-,39-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective vasopressin V1A receptor antagonist. Inhibits vasopressin and oxytocin-induced increases in intracellular calcium concentrations in vitro (IC50 values are 5 and 30 nM respectively). Exhibits potent and prolonged antivasopressor activity and induces anxiolytic-like effects in the dorsal, but not ventral, hippocampus in vivo. |

(d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin Dilution Calculator

(d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- Atractylenolide II
Catalog No.:BCN1044
CAS No.:73069-14-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
Dissociation of the anxiolytic-like effects of Avpr1a and Avpr1b receptor antagonists in the dorsal and ventral hippocampus.[Pubmed:18508119]
Neuropeptides. 2008 Aug;42(4):411-21.
Arginine-vasopressin (AVP) is synthesized and released centrally in several brain structures. AVP is thought to mediate anxiety-related behavior through two central receptor subtypes, Avpr1a and Avpr1b. Although these AVP receptor subtypes are expressed in several brain regions, including the hippocampus, little is known about their explicit role in unconditioned fear or anxiety. This experiment assessed the anxiety-related effects of a selective Avpr1a antagonist ([beta-Mercapto-beta,beta-cyclopentamethylenepropionyl1, O-me-Tyr2, Arg8]-AVP) and a selective Avpr1b antagonist ((2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo -2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide; SSR 149415) microinfused into either the dorsal or ventral sub-regions of the rat hippocampus. Avpr1a antagonism in the ventral, but not the dorsal hippocampus reduced rats' anxiety-like behavior in the elevated plus-maze test. Conversely, Avpr1b antagonism in the dorsal, but not the ventral, hippocampus reduced anxiety in the plus-maze test. Neither antagonist reduced anxiety-like behavior in the shock-probe burying test. Overall, the results show that both receptor subtypes of AVP are involved in anxiety-related responses, but their specific contributions depend on three variables: (1) the anxiety-related response (shock-probe avoidance versus open-arm avoidance), (2) the receptor subtype antagonized (Avpr1a versus Avpr1b), and (3) the area of hippocampus (dorsal versus ventral) into which these antagonists are infused. These dissociations suggest that different fear responses are under the control of specific AVP receptor systems within discrete parts of the hippocampus.
Vasopressin inhibits sarcolemmal ATP-sensitive K+ channels via V1 receptors activation in the guinea pig heart.[Pubmed:11922278]
Circ J. 2002 Mar;66(3):277-82.
To examine the effect of vasopressin on the sarcolemmal ATP-sensitive K (K(ATP)) channel, cell-attached, insideout and open-cell-attached methods of patch clamp techniques were used in isolated guinea pig ventricular myocytes. Suppressing both glycolytic and oxidative ATP production attained K(ATP) channel activation. In the cell-attached mode, vasopressin inhibited KATP channels in a concentration-dependent manner with an IC50 of 15.1+/-1.8 nmol/L. In the inside-out configuration, vasopressin failed to block K(ATP) channels. In the cell-attached mode, manning compound (1 micromol/L), a V1 receptor-selective antagonist, blocked the inhibitory action of vasopressin, although OPC-31260 (1 micromol/L), a V2 receptor-selective antagonist could not affect the action of vasopressin. In addition, vasopressin lost its inhibitory action on K(ATP) channels when the channel was activated by pinacidil, a K channel opener and in the open-cell-attached mode effected by streptolysin-O. Thus, the inhibitory action of vasopressin K(ATP) channels may occur via V1 receptor related mechanism.
Arginine vasopressin and oxytocin increase intracellular calcium and cAMP in human glomerular epithelial cells in culture.[Pubmed:8871886]
Kidney Blood Press Res. 1996;19(2):81-6.
The signal transduction linkages of arginine vasopressin (AVP) and oxytocin receptors were investigated in human glomerular epithelial cells (GEC) in culture. AVP (ED50, 10(-7) mol/l) and oxytocin (ED50, 3 x 10(-8) mol/l) induced a rapid, transient and dose-dependent increase in [Ca2+]i as detected by fura-2 microfluorimetry. The baseline of [Ca2+]i in human GEC was 109 +/- 2.8 nmol/l (n = 60). The V1a receptor antagonist [d(CH2)5(1), Tyr(Me)2, Arg8]-vasopressin inhibited the AVP-(IC50, 5 x 10(-9) mol/l) and oxytocin-induced (IC50, 3 x 10(-8) mol/l) increase in [Ca2+]i in a dose-dependent manner. Both, AVP and oxytocin caused accumulation of cAMP. The AVP-stimulated cAMP increase was blocked by pretreatment of human GEC with the V1a receptor antagonist (10(-7) mol/l), whereas the oxytocin-induced cAMP accumulation remained uninfluenced. In conclusion the present results indicate that: (1) V1a receptor activation, AVP and oxytocin induce a transient elevation in [Ca2+]i in human GEC; (2) AVP and oxytocin cause cAMP accumulation; (3) the AVP-induced cAMP accumulation is inhibited by a V1a receptor antagonist, whereas (4) the oxytocin response showed no effect. In addition, a different receptor might be possible, at least in oxytocin-induced-cAMP accumulation.


