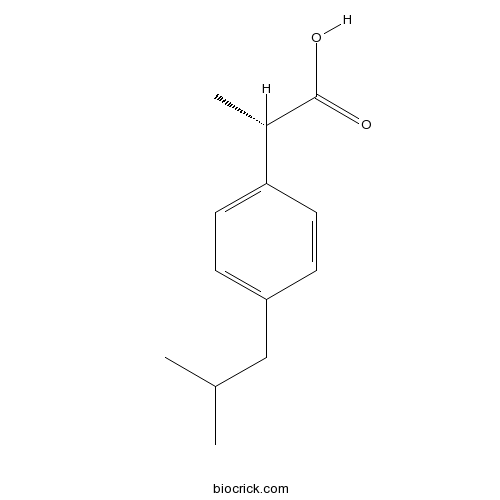
(S)-(+)-IbuprofenCyclooxygenase inhibitor (COX-1 > COX-2) CAS# 51146-56-6 |
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 51146-56-6 | SDF | Download SDF |
| PubChem ID | 39912 | Appearance | Powder |
| Formula | C13H18O2 | M.Wt | 206.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (S)-Ibuprofen | ||
| Solubility | DMSO : 100 mg/mL (484.78 mM; Need ultrasonic) Ethanol : 100 mg/mL (484.78 mM; Need ultrasonic) H2O : 1 mg/mL (4.85 mM; Need ultrasonic and warming) | ||
| Chemical Name | (2S)-2-[4-(2-methylpropyl)phenyl]propanoic acid | ||
| SMILES | CC(C)CC1=CC=C(C=C1)C(C)C(=O)O | ||
| Standard InChIKey | HEFNNWSXXWATRW-JTQLQIEISA-N | ||
| Standard InChI | InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)/t10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-steroidal anti-inflammatory drug (NSAID) that inhibits cyclooxygenase 1 and cyclooxygenase 2 (IC50 values are 12 and 80 μM respectively). Active isomer of ibuprofen. |

(S)-(+)-Ibuprofen Dilution Calculator

(S)-(+)-Ibuprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8478 mL | 24.2389 mL | 48.4778 mL | 96.9556 mL | 121.1945 mL |
| 5 mM | 0.9696 mL | 4.8478 mL | 9.6956 mL | 19.3911 mL | 24.2389 mL |
| 10 mM | 0.4848 mL | 2.4239 mL | 4.8478 mL | 9.6956 mL | 12.1194 mL |
| 50 mM | 0.097 mL | 0.4848 mL | 0.9696 mL | 1.9391 mL | 2.4239 mL |
| 100 mM | 0.0485 mL | 0.2424 mL | 0.4848 mL | 0.9696 mL | 1.2119 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
S(+)-Ibuprofen is capable of inhibiting cyclooxygenase (COX) at clinically relevant concentrations, R(-)-ibuprofen is not a COX inhibitor.
- (+)-trans-Isolimonene
Catalog No.:BCC9236
CAS No.:5113-87-1
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- 8-Bromo-cGMP, sodium salt
Catalog No.:BCC6935
CAS No.:51116-01-9
- Gitogenin
Catalog No.:BCN3886
CAS No.:511-96-6
- Plumieride
Catalog No.:BCN5631
CAS No.:511-89-7
- Vitamin D4
Catalog No.:BCC2042
CAS No.:511-28-4
- Totarol
Catalog No.:BCN4627
CAS No.:511-15-9
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
- alpha-Onocerol
Catalog No.:BCN5630
CAS No.:511-01-3
- Nicotine 1'-N-oxide
Catalog No.:BCN6892
CAS No.:51095-86-4
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- (R)-(-)-Ibuprofen
Catalog No.:BCC4062
CAS No.:51146-57-7
- Episyringaresinol
Catalog No.:BCN7023
CAS No.:51152-20-6
- Boc-D-Asp(OBzl)-OH
Catalog No.:BCC3371
CAS No.:51186-58-4
- Withaferin A
Catalog No.:BCC7495
CAS No.:5119-48-2
- Diosgenin
Catalog No.:BCN6272
CAS No.:512-04-9
- Yamogenin
Catalog No.:BCN8277
CAS No.:512-06-1
- Raffinose
Catalog No.:BCN8427
CAS No.:512-69-6
- Ascaridole
Catalog No.:BCC8121
CAS No.:512-85-6
- Boc-Arg(Z)-OH
Catalog No.:BCC3068
CAS No.:51219-18-2
- 6,8-Diprenylgenistein
Catalog No.:BCN4805
CAS No.:51225-28-6
- Wighteone
Catalog No.:BCN5632
CAS No.:51225-30-0
- N-Phthaloyl-Phe-OH
Catalog No.:BCC3016
CAS No.:5123-55-7
S(+)-ibuprofen destabilizes MYC/MYCN and AKT, increases p53 expression, and induces unfolded protein response and favorable phenotype in neuroblastoma cell lines.[Pubmed:24173829]
Int J Oncol. 2014 Jan;44(1):35-43.
Neuroblastoma is a common pediatric solid tumor that exhibits a striking clinical bipolarity: favorable and unfavorable. The survival rate of children with unfavorable neuroblastoma remains low among all childhood cancers. MYCN and MYC play a crucial role in determining the malignancy of unfavorable neuroblastomas, whereas high-level expression of the favorable neuroblastoma genes is associated with a good disease outcome and confers growth suppression of neuroblastoma cells. A small fraction of neuroblastomas harbors TP53 mutations at diagnosis, but a higher proportion of the relapse cases acquire TP53 mutations. In this study, we investigated the effect of S(+)-ibuprofen on neuroblastoma cell lines, focusing on the expression of the MYCN, MYC, AKT, p53 proteins and the favorable neuroblastoma genes in vitro as biomarkers of malignancy. Treatment of neuroblastoma cell lines with S(+)-ibuprofen resulted in a significant growth suppression. This growth effect was accompanied by a marked decrease in the expression of MYC, MYCN, AKT and an increase in p53 expression in neuroblastoma cell lines without TP53 mutation. In addition, S(+)-ibuprofen enhanced the expression of some favorable neuroblastoma genes (EPHB6, CD44) and genes involved in growth suppression and differentiation (EGR1, EPHA2, NRG1 and SEL1L). Gene expression profile and Ingenuity pathway analyses using TP53-mutated SKNAS cells further revealed that S(+)-ibuprofen suppressed molecular pathways associated with cell growth and conversely enhanced those of cell cycle arrest and the unfolded protein response. Collectively, these results suggest that S(+)-ibuprofen or its related compounds may have the potential for therapeutic and/or palliative use for unfavorable neuroblastoma.
Impact of patent ductus arteriosus and subsequent therapy with ibuprofen on the release of S-100B and oxidative stress index in preterm infants.[Pubmed:25542161]
Med Sci Monit. 2014 Dec 26;20:2799-805.
BACKGROUND: Hemodynamically significant patent ductus arteriosus (hsPDA) leads to injury in tissues/organs by reducing perfusion of organs and causing oxidative stress. The purpose of this study was to evaluate the oxidant/antioxidant status in preterm infants with hsPDA by measuring the total antioxidant capacity and total oxidant status and to assess neuronal damage due to oxidant stress related to hsPDA. MATERIAL AND METHODS: This prospective study included 37 low-birth-weight infants with echocardiographically diagnosed hsPDA treated with oral ibuprofen and a control group of 40 infants without PDA. Blood samples were taken from all infants, and than the total antioxidant capacity (TAC), total oxidant status (TOS), and S-100B protein levels were assessed and oxidative stress index was calculated before and after therapy. RESULTS: The mean pre-therapy TOS level and oxidative stress index (OSI) value of the patients with hsPDA were significantly higher, but TAC level was lower than in the control group. There were no statistically significant differences in the mean post-therapy values of TOS, TAC, OSI, and S-100B protein between the two groups. CONCLUSIONS: hsPDA may cause cellular injury by increasing oxidative stress and damaging tissue perfusion; however the brain can compensate for oxidative stress and impaired tissue perfusion through well-developed autoregulation systems to decrease tissue injury.
In vitro evaluation of S-(+)-ibuprofen as drug candidate for intra-articular drug delivery system.[Pubmed:24168233]
Drug Dev Ind Pharm. 2015 Jan;41(1):85-94.
Intra-articular drug delivery systems (DDSs) are envisaged as interesting alternative to locally release non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen to reduce pain in patients with osteoarthritis. The present study examines the efficacy of S-(+)-ibuprofen on cartilage degradation as drug candidate for DDS loading. Humeral cartilage and joint capsule explants were collected from healthy sheep shoulder joints and they were cultured in mono- or in co-culture for 13 days with LPS in combination with S-(+)-ibuprofen at 50 microM and 1 mM. S-(+)-ibuprofen (50 microM) blocked prostaglandins production in LPS-activated explants but did not reduce cartilage degradation. By contrast, 1 mM S-(+)-ibuprofen treatment of cartilage explants reduced nitric oxide synthesis by 51% (p = 0.0072), proteoglycans degradation by 35% (p = 0.0114) and expression of serum amyloid protein - the main protein induced upon LPS challenge - by 44% (p < 0.0001). On contrary, in presence of synovial membrane, the protective effects of S-(+)-ibuprofen on cartilage damages were significantly diminished. At 1mM, S-(+)-ibuprofen reduced the cell lysis during culture of cartilage and joint capsule either in mono- or in co-culture. This study performed on sheep explants shows that 1 mM S-(+)-ibuprofen inhibited cartilage degradation via a mechanism independent of cyclooxygenase inhibition. Reduction of prostaglandins synthesis at 50 microM in all treatment groups and reduction of cartilage degradation observed at 1 mM suggest that S-(+)-ibuprofen could be considered as a promising drug candidate for the loading of intra-articular DDS.
Two Enzyme Cooperatively Catalyzed Tandem Polymerization for the Synthesis of Polyester Containing Chiral (R)- or (S)-Ibuprofen Pendants.[Pubmed:25200738]
Macromol Rapid Commun. 2014 Sep 8.
An interesting cooperation between Candida antarctica Lipase B (CAL-B) and alkaline protease from Bacillus subtilis (BSP) in the copolymerization of bulky ibuprofen-containing hydroxyacid methyl ester (HAEP) and epsilon-caprolactone (epsilon-CL) is observed. This cooperation improved the M n of the polymers from 3130 (CAL-B) to 9200 g mol(-1) (CAL-B/BSP). Experimental results clearly indicate that CAL-B mainly catalyzes the ring-opening polymerization (ROP) of epsilon-CL under the initiation of HAEP to form the homopolymer of epsilon-CL, while BSP catalyzes the subsequent polycondensation of the ROP product to yield the copolymer with increased molecular weight. Furthermore, using suitable chemo-enzymatic methods, valuable polyesters with chiral (R)- or (S)-ibuprofen pendants can be tailor-made.


