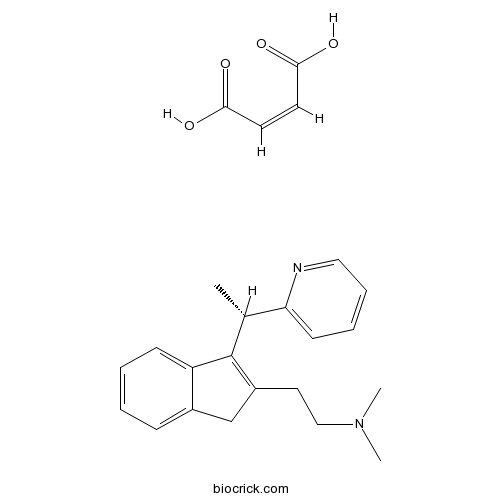
(S)-(+)-Dimethindene maleateH1 antagonist. Also M2 muscarinic antagonist CAS# 136152-65-3 |
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
- Tivantinib (ARQ 197)
Catalog No.:BCC3688
CAS No.:905854-02-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 136152-65-3 | SDF | Download SDF |
| PubChem ID | 56972160 | Appearance | Powder |
| Formula | C24H28N2O4 | M.Wt | 408.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water with gentle warming | ||
| Chemical Name | (Z)-but-2-enedioic acid;N,N-dimethyl-2-[3-[(1S)-1-pyridin-2-ylethyl]-1H-inden-2-yl]ethanamine | ||
| SMILES | CC(C1=CC=CC=N1)C2=C(CC3=CC=CC=C32)CCN(C)C.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | SWECWXGUJQLXJF-HFNHQGOYSA-N | ||
| Standard InChI | InChI=1S/C20H24N2.C4H4O4/c1-15(19-10-6-7-12-21-19)20-17(11-13-22(2)3)14-16-8-4-5-9-18(16)20;5-3(6)1-2-4(7)8/h4-10,12,15H,11,13-14H2,1-3H3;1-2H,(H,5,6)(H,7,8)/b;2-1-/t15-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Enantiomer that is a subtype-selective M2 muscarinic receptor antagonist (pKi values are 7.08, 7.78, 6.70 and 7.00 for M1, M2, M3 and M4 receptors respectively). Also H1 histamine receptor antagonist (pKi = 7.48). Allows formation of extended pluripotent stem (EPS) cells in combination with CHIR 99021 (Cat.No. 4423), minocycline hydrochloride (Cat.No. 3268) and human leukemia inhibitory factor. |

(S)-(+)-Dimethindene maleate Dilution Calculator

(S)-(+)-Dimethindene maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.448 mL | 12.2399 mL | 24.4798 mL | 48.9596 mL | 61.1995 mL |
| 5 mM | 0.4896 mL | 2.448 mL | 4.896 mL | 9.7919 mL | 12.2399 mL |
| 10 mM | 0.2448 mL | 1.224 mL | 2.448 mL | 4.896 mL | 6.12 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9792 mL | 1.224 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Minocycline HCl
Catalog No.:BCC4679
CAS No.:13614-98-7
- 3,7-Di-O-methylducheside A
Catalog No.:BCN6191
CAS No.:136133-08-9
- Przewalskinic acid A
Catalog No.:BCN2925
CAS No.:136112-75-9
- LDC1267
Catalog No.:BCC5577
CAS No.:1361030-48-9
- 14-Dehydrodelcosine
Catalog No.:BCN8119
CAS No.:1361-18-8
- Isomucronulatol 7-O-beta-glucoside
Catalog No.:BCN8088
CAS No.:136087-29-1
- Lobetyolin
Catalog No.:BCN5894
CAS No.:136085-37-5
- Onjixanthone II
Catalog No.:BCN7559
CAS No.:136083-93-7
- 5-Methoxyisolariciresinol
Catalog No.:BCN7016
CAS No.:136082-41-2
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- 9-Dehydroxyeurotinone
Catalog No.:BCN7397
CAS No.:1360606-85-4
- Desrhamnosylmartynoside
Catalog No.:BCN7648
CAS No.:136055-64-6
- E3330
Catalog No.:BCC6421
CAS No.:136164-66-4
- INNO-206
Catalog No.:BCC1651
CAS No.:1361644-26-9
- Lobetyol
Catalog No.:BCN3321
CAS No.:136171-87-4
- 6-O-Caffeoylarbutin
Catalog No.:BCN6192
CAS No.:136172-60-6
- Sarranicine
Catalog No.:BCN2025
CAS No.:136173-25-6
- Neosarracine
Catalog No.:BCN2026
CAS No.:136173-26-7
- Neosarranicine
Catalog No.:BCN2024
CAS No.:136173-27-8
- PALDA
Catalog No.:BCC7287
CAS No.:136181-87-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- 3,4'-Dihydroxy-3',5'-dimethoxypropiophenone
Catalog No.:BCN1579
CAS No.:136196-47-9
- KW 3902
Catalog No.:BCC6124
CAS No.:136199-02-5
- Absinthiin
Catalog No.:BCN2314
CAS No.:1362-42-1
Neuronal soma-dendritic and prejunctional M1-M4 receptors in gastrointestinal and genitourinary smooth muscle.[Pubmed:10069503]
Life Sci. 1999;64(6-7):403-10.
A variety of neurons in gastrointestinal and genitourinary smooth muscle express muscarinic auto- as well as heteroreceptors. These receptors are found on the soma and dendrites of many cholinergic, sympathetic and NANC neurons and on axon terminals. A given neuron may contain both excitatory and inhibitory presynaptic muscarinic receptors. The subtypes involved are species- and tissue-dependent, and neuronal M1 to M4 receptors have been shown to be expressed in smooth muscle tissues. In this study, the ability of several selective muscarinic receptor antagonists to inhibit the effect of arecaidine propargyl ester (APE) on prejunctional muscarinic receptors on sympathetic nerve endings in the rabbit anococcygeus muscle (RAM) was investigated to characterise the receptor subtype involved. Electrical field stimulation (EFS) resulted in a release of noradrenaline (NA) eliciting monophasic contractions due to stimulation of postjunctional alpha1-adrenoceptors. The selective muscarinic agonist APE did not reduce contractions to exogenous NA, but caused a concentration-related and N-methylatropine-sensitive inhibition of neurogenic responses. All muscarinic antagonists investigated failed to affect the EFS-induced contractions, but shifted the concentration-response curve of APE to the right in a parallel and surmountable fashion. Schild analysis yielded regression lines of unit slope, indicating competitive antagonism. The following rank order of antagonist potencies (pA2 values) was found: tripitramine (9.10) > AQ-RA 741 (8.26) > or = himbacine (8.04) > or = (S)-dimethindene (7.69) > pirenzepine (6.46) > or = p-F-HHSiD (6.27). A comparison of the pA2 values determined in the present study with literature binding and functional affinities obtained at native or recombinant M1 to M5 receptors strongly suggests that NA release from sympathetic nerve endings in RAM is inhibited by activation of prejunctional muscarinic M2 receptors.
The (S)-(+)-enantiomer of dimethindene: a novel M2-selective muscarinic receptor antagonist.[Pubmed:8608784]
Eur J Pharmacol. 1995 Nov 24;286(3):229-40.
The present study was designed to determine to determine the in vitro affinity profile of (R)-(-)-dimethindene and (S)-(+)-dimethindene at muscarinic receptor subtypes using both functional and binding assays. In addition, the racemate was investigated in functional studies. The functional muscarinic receptors studied were putative M1 receptors in rabbit vas deferens and rat duodenum, M2 receptors in guinea-pig left atria and rabbit vas deferens, as well as M3 receptors in guinea-pig ileum and trachea. Furthermore, the histamine H1 antagonism by (R)-(-)- and (S)-(+)-dimethindene has been examined in guinea-pig ileum. Muscarinic binding selectivity was assessed in homogenates from human neuroblastoma NB-OF 1 cells (M1), rat heart (M2), pancreas (3) and striatum (M4). The results demonstrate that (S)-(+)-dimethindene is a potent M2-selective muscarinic receptor antagonist (pA2 = 7.86/7.74; pKi = 7.78) with lower affinities for the muscarinic M1 (pA2 = 6.83/6.36; pKi = 7.08), the M3 (pA2 = 6.92/6.96; pKi = 6.70) and the M4 receptors (pKi = 7.00), respectively. The (S)-(+)-enantiomer was more potent (up to 41-fold) than the (R)-(-)-enantiomer in all muscarinic assays. In contrast, the stereoselectivity was inverse at histamine H1 receptors, the (R)-(-)-enantiomer being the eutomer (pA2 = 9.42; pA2/(S)-isomer = 7.48). In conclusion, (S)-(+)-dimethindene is a useful tool to investigate muscarinic receptor heterogeneity. In addition, this lipophilic compound might become the starting point for the development of M2-selective muscarinic receptor antagonists useful as diagnostic tools for quantifying muscarinic M2 receptors in the central nervous system with positron emission tomography imaging, and to test the hypothesis that muscarinic M2 receptor antagonists show beneficial effects in the treatment of cognitive disorders.


