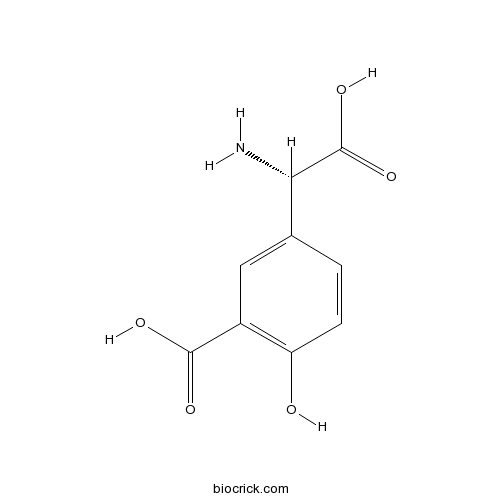
(S)-3-Carboxy-4-hydroxyphenylglycineSelective group II mGlu agonist, also group I mGlu antagonist CAS# 55136-48-6 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 55136-48-6 | SDF | Download SDF |
| PubChem ID | 5311460 | Appearance | Powder |
| Formula | C9H9NO5 | M.Wt | 211.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (<em>S</em>)-3C4HPG | ||
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | 5-[(S)-amino(carboxy)methyl]-2-hydroxybenzoic acid | ||
| SMILES | C1=CC(=C(C=C1C(C(=O)O)N)C(=O)O)O | ||
| Standard InChIKey | CHZBCZTXSTWCIG-ZETCQYMHSA-N | ||
| Standard InChI | InChI=1S/C9H9NO5/c10-7(9(14)15)4-1-2-6(11)5(3-4)8(12)13/h1-3,7,11H,10H2,(H,12,13)(H,14,15)/t7-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mixed group I metabotropic glutamate receptor antagonist and group II mGlu agonist. (R)-3-Carboxy-4-hydroxyphenylglycine also available. |

(S)-3-Carboxy-4-hydroxyphenylglycine Dilution Calculator

(S)-3-Carboxy-4-hydroxyphenylglycine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7355 mL | 23.6776 mL | 47.3552 mL | 94.7104 mL | 118.388 mL |
| 5 mM | 0.9471 mL | 4.7355 mL | 9.471 mL | 18.9421 mL | 23.6776 mL |
| 10 mM | 0.4736 mL | 2.3678 mL | 4.7355 mL | 9.471 mL | 11.8388 mL |
| 50 mM | 0.0947 mL | 0.4736 mL | 0.9471 mL | 1.8942 mL | 2.3678 mL |
| 100 mM | 0.0474 mL | 0.2368 mL | 0.4736 mL | 0.9471 mL | 1.1839 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4beta-Carboxy-19-nortotarol
Catalog No.:BCN4065
CAS No.:55102-39-1
- 2'-Aminoacetophenone
Catalog No.:BCN1746
CAS No.:551-93-9
- Dimetridazole
Catalog No.:BCC8944
CAS No.:551-92-8
- Supinine
Catalog No.:BCN1952
CAS No.:551-58-6
- Viridiflorine
Catalog No.:BCN2045
CAS No.:551-57-5
- 6-Aminopenicillanic acid
Catalog No.:BCC8765
CAS No.:551-16-6
- Liquiritin
Catalog No.:BCN5944
CAS No.:551-15-5
- 3-Butylidenephthalide
Catalog No.:BCN6345
CAS No.:551-08-6
- EVP-6124
Catalog No.:BCC1566
CAS No.:550999-75-2
- EVP-6124 hydrochloride
Catalog No.:BCC1567
CAS No.:550999-74-1
- Nalmefene - d3
Catalog No.:BCC6093
CAS No.:55096-26-9
- 5,6,7-Trimethoxycoumarin
Catalog No.:BCN7590
CAS No.:55085-47-7
- Kaempferol 3-sophoroside-7-glucoside
Catalog No.:BCN7825
CAS No.:55136-76-0
- Oxohydrastinine
Catalog No.:BCN3299
CAS No.:552-29-4
- Paeonol
Catalog No.:BCN5738
CAS No.:552-41-0
- Isorhoifolin
Catalog No.:BCN5739
CAS No.:552-57-8
- Eriodictyol
Catalog No.:BCN1209
CAS No.:552-58-9
- Prunetin
Catalog No.:BCN2335
CAS No.:552-59-0
- Daidzin
Catalog No.:BCN5891
CAS No.:552-66-9
- Sasapyrine
Catalog No.:BCC4714
CAS No.:552-94-3
- Dexamethasone Sodium Phosphate
Catalog No.:BCC4557
CAS No.:55203-24-2
- AP 18
Catalog No.:BCC7634
CAS No.:55224-94-7
- Rolapitant
Catalog No.:BCC6441
CAS No.:552292-08-7
- Vallesiachotamine
Catalog No.:BCN3548
CAS No.:5523-37-5
Stimulation of high-affinity GTPase activity through group II metabotropic glutamate receptors in rat hippocampal and striatal membranes.[Pubmed:11202611]
Jpn J Pharmacol. 2000 Dec;84(4):399-404.
The stimulation of high-affinity GTPase activity through metabotropic glutamate receptors (mGluRs) was pharmacologically characterized with the use of a series of agonists for mGluRs in rat hippocampal and striatal membranes. The pharmacological profile of the response was almost identical to each other between both brain regions. Thus, the high-affinity GTPase activities were stimulated by several mGluR-related compounds with the following rank order of potency: (2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)glycine (DCG-IV) = (2S,1'S,2'S)-2-(carboxycyclopropyl)glycine (L-CCG-I) > L-glutamate = 2R,4R-4-aminopyrrolidine-2,4-dicarboxylate [(2R,4R)-APDC] > (S)-4-carboxy-3-hydroxyphenylglycine [(S)-4C3HPG] = 1S,3R-1-aminocyclopentane-1,3-dicarboxylate [(1S,3R)-ACPD] > (S)-3-Carboxy-4-hydroxyphenylglycine [(S)-3C4HPG] = ibotenate. The negative logarithmically transformed EC50 (pEC50) values of these compounds in both brain regions were significantly correlated with those reported previously in the cerebral cortical membranes (N. Nishi et al., Br. J. Pharmacol., 130, 1664-1670, 2000). On the contrary, other reagents including a selective group I mGluRs agonist, (RS)-3,5-dihydroxyphenylglycine [(RS)-3,5-DHPG], and selective group III mGluRs agonists such as L(+)-2-amino-4-phosphonobutylate (L-AP4) and L-serine-O-phosphate (L-SOP) had little or no effects even at the highest concentration examined. Quisqualate was also a very weak agonist in both regions. These results indicate that mGluR-mediated high-affinity GTPase activity derives from the Gi proteins associated with adenylyl cyclase inhibition through group II mGluRs, in particular the mGluR2 subtype, in rat hippocampal and striatal membranes.
Pharmacological characterization of metabotropic glutamate receptor-mediated high-affinity GTPase activity in rat cerebral cortical membranes.[Pubmed:10928972]
Br J Pharmacol. 2000 Aug;130(7):1664-70.
Activation of heterotrimeric guanine nucleotide-binding regulatory proteins (G-proteins) functionally coupled to metabotropic glutamate receptors (mGluRs) was assessed by agonist-induced high-affinity GTPase (EC3.6.1.-) activity in rat cerebral cortical membranes. L-Glutamate (1 mM) stimulated high-affinity GTPase activity to the same extent throughout the incubation period up to 20 min, in a Mg(2+)-dependent manner. The addition of 1 mM L-glutamate augmented V(max) of the enzyme activity (1670 to 3850 pmol mg(-1) protein 15 min(-1)) with slight increase in K(M) value (0.26 to 0.63 microM). The high-affinity GTPase activity was stimulated by the following compounds with a rank order of potency of (2S,2'R,3'R)-2-(2', 3'-dicarboxycyclopropyl) glycine (DCG-IV) > (2S,1'S, 2'S)-2-(carboxycyclopyropyl)glycine (L-CCG-I) > L-glutamate > or = 2R, 4R-4-aminopyrrolidine-2,4-dicarboxylate [(2R,4R)-APDC] > 1S, 3R-1-aminocyclopentane-1,3-dicarboxylate [(1S,3R)-ACPD] > (S)-4-carboxy-3-hydroxyphenylglycine [(S)-4C3HPG] > (S)-3-Carboxy-4-hydroxyphenylglycine [(S)-3C4HPG] > ibotenate, but not by L-(+)-2-amino-4-phosphonobutyrate (L-AP4), (RS)-3, 5-dihydroxyphenylglycine [(RS)-3,5-DHPG], quisqualate, or L-serine-O-phosphate (L-SOP), indicative of involvement of group II mGluRs, in particular mGluR2. (2S)-alpha-Ethylglutamate (EGLU), a presumably selective antagonist against group II mGluRs, inhibited DCG-IV-stimulated high-affinity GTPase activity in a competitive manner with an apparent K(B) of 220 microM. L-Glutamate-stimulated activity was eliminated by pretreatment of the membranes with sulfhydryl alkylating agent N-ethylmaleimide (NEM) at 30-50 microM, indicating that G-proteins of the G(i) family are involved. These results indicate that mGluR agonist-induced high-affinity GTPase activity in rat cerebral cortical membranes may be used to detect the functional interaction between group II mGluRs, in particular mGluR2, and NEM-sensitive G(i) proteins.
Regional distribution and pharmacological characteristics of [3H]N-acetyl-aspartyl-glutamate (NAAG) binding sites in rat brain.[Pubmed:10913688]
Neurochem Int. 2001 Jan;38(1):53-62.
Autoradiographical studies revealed that 10 nM [3H]N-acetyl-aspartyl-glutamate (NAAG) labelled grey matter structures, particularly in the hippocamus, cerebral neocortex, striatum, septal nuclei and the cerebellar cortex. The binding was inhibited by (2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)-glycine (DCG IV), an agonist at group II metabotropic glutamate receptors (mGluR II). (RS)-alpha-Methyl-4-tetrazolylphenylglycine (MTPG), (RS)-alpha-cyclopropyl-4-phosphonoglycine (CPPG) and (RS)-alpha-methylserine-O-phosphate monophenyl ester (MSOPPE), all antagonists at mGluR II and mGluR III, also inhibited [3H]NAAG binding. Other inhibitors were (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate (ACPD), a broad-spectrum mGluR agonist with preference for groups I and II and the mGluR I agonists/mGluR II antagonists (S)-3-Carboxy-4-hydroxyphenylglycine (3,4-CHPG) and (S)-4-carboxy-3-hydroxyphenylglycine (4,3-CHPG). Neither the mGluR I specific agonist (S)-dihydroxyphenylglycine nor any of the ionotropic glutamate receptor ligands such as kainate, AMPA and MK-801 had strong effects (except for the competitive NMDA antagonist CGS 19755, which produced 20-40% inhibition at 100 microM) suggesting that, at low nM concentrations, [3H]NAAG binds predominantly to metabotropic glutamate receptors, particularly those of the mGluR II type. Several studies have indicated that NAAG can interact with mGluR II and the present study supports this notion by demonstrating that sites capable of binding NAAG at low concentrations and displaying pharmacological characteristics of mGluR II exist in the central nervous tissue. Furthermore, the results show that autoradiography of [3H]NAAG binding can be used to quantify the distribution of such sites in distinct brain regions and study their pharmacology at the same time.
The enhancement and the inhibition of noradrenaline-induced cyclic AMP accumulation in rat brain by stimulation of metabotropic glutamate receptors.[Pubmed:8843491]
Prog Neuropsychopharmacol Biol Psychiatry. 1996 May;20(4):673-90.
1. The actions of several metabotropic glutamate receptor and antagonists on noradrenaline (NA)-stimulated [3H]-cyclic AMP accumulation were investigated in rat cerebral cortical slices. 2. Quisqualate (QUIS), L-2-amino-3-phosphonopropionic acid (L-AP3) and glutamate (GLU) elicited concentration-dependent inhibition of (NA)-stimulated [3H]-cyclic AMP accumulation, with IC50 values of 105 +/- 29, 275 +/- 36 and 944 +/- 150 microM respectively. In contrast (Rs)-alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) (0.5 mM) and N-methyl-D-aspartic acid (NMDA) (0.5 mM) had no effect. 3. (2S,3S,4S)-alpha-(Carboxycyclopropyl)glycine (L-CCGI), 1-Aminocyclo-pentane-1S,3R-dicarbo-xylate (1S,3R-ACPD), ibotenate (IBO) and (RS)-4-carboxy-3-hydroxy-phenylglycine (CHPG)elicited a concentration-dependent enhancement of NA-stimulated [3H]-cyclic AMP accumulation, with EC50 values of 2.5 +/- 0.11, 42 +/- 1.3, 97.8 +/- 2.1 and 157 +/- 13.4 microM, respectively. 4. (S)-3-Carboxy-4-hydroxyphenylglycine (3C4HPG) and (S)-4-carboxy-3-hydroxyphenyl-glycine (4C3HPG) produced a biphasic effect, at concentrations up to 100 and 500 microM, respectively, they significantly enhanced the action of NA (100 microM), at 1mM concentration both compounds as well as alpha-methyl-4-carboxyphenylglycine (MCPG) produced a significant inhibition of NA-stimulated cyclic AMP accumulation. 5. A putative mGluR antagonist-L-AP3, inhibited the 1S,3R-ACPD (100 microM) induced enhancement of the action of NA (100 microM) on [3H]-cyclic AMP accumulation in a biphasic manner with an IC50 of 4.5 microM for the high affinity site, which represented 65% of the total and an IC50 of 283 microM for the low affinity site. 6. beta-adrenoceptor antagonist propranolol inhibited the interaction between 1S,3R-ACPD (100 microM) and NA (100 microM) on [3H]-cyclic AMP accumulation by about 80%, with an IC50 of 0.52 +/- 0.011 microM, to the level observed after 1S,3R-ACPD alone. Prazosin, an alpha 1-adrenoceptor antagonist was more potent (IC50 of 0.091 +/- 0.012 microM) but less efficacious (60% inhibition) as an inhibitor of the interaction either between NA and 1S,3R-ACPD while yohimbine, na alpha 2-adrenoceptor antagonist (up to 1 microM) had no effect. 7. Neither the protein kinase C inhibitor - staurosporine (10 microM) nor thapsigargin (1 microM), which depletes IP3 sensitive calcium stores, inhibited significantly the 1S,3R-ACPD (100 microM)-induced enhancement of the action of NA (100 microM) on [3H]-cyclic AMP accumulation. 8. Adenosine deaminase (0.5 U/ml) abolished both the 1S,3R-ACPD (100 microM)-induced [3H]-cyclic AMP accumulation and the synergistic interaction of this compound with NA (100 microM). 9. These results indicate the existence of different subtypes of metabotropic glutamate receptors in rat brain which either inhibit or enhance the NA-stimulated [3H]-cyclic AMP accumulation. The enhancement in cerebral cortical slices is mediated via receptors which are blocked with high affinity by L-AP3 and occurs via interactions with endogenous adenosine; the inhibition is mediated by receptors sensitive to quisqualate, L-AP3 and glutamate and may represent a predominant interaction between NA and excitatory amino acids (EAA), which in cerebral cortical slices is masked by excitatory effects.
Actions of phenylglycine analogs at subtypes of the metabotropic glutamate receptor family.[Pubmed:7515823]
Eur J Pharmacol. 1994 Mar 15;267(1):77-84.
The functional effects of phenylglycine analogs on metabotropic glutamate receptor (mGluR) subtypes mGluR1 alpha, mGluR2 and mGluR4 were examined. (S)-4-Carboxyphenylglycine (IC50 = 65 +/- 5 microM), (R,S)-alpha-methyl-4-carboxyphenylglycine (IC50 = 155 +/- 38 microM) and (S)-3-Carboxy-4-hydroxyphenylglycine (IC50 = 290 +/- 47 microM) competitively antagonized glutamate-stimulated phosphoinositide hydrolysis in baby hamster kidney (BHK) cells stably expressing mGluR1 alpha. (S)-4-Carboxyphenylglycine and (R,S)-alpha-methyl-4-carboxyphenylglycine competitively antagonized glutamate-induced inhibition of forskolin-stimulated cAMP-formation in BHK cells stably expressing mGluR2 with IC50 values of 577 +/- 74 microM and 340 +/- 59 microM, respectively. (R,S)-4-carboxy-3-hydroxyphenylglycine, (R)-3-hydroxyphenylglycine and (S)-3-Carboxy-4-hydroxyphenylglycine were agonists at mGluR2 with EC50 values of 48 +/- 5 microM, 451 +/- 93 and 97 +/- 12 microM, respectively. In parallel experiments, no activities of these phenylglycine analogs at mGluR4 were observed. The present report demonstrates that phenylglycine analogs possess differential functional activities at subtypes of the mGluR family.
Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptor subtypes.[Pubmed:8730745]
Br J Pharmacol. 1996 Apr;117(7):1493-503.
1. We investigated the agonist and antagonist activities of 22 new phenylglycine and phenylalanine derivatives for metabotropic glutamate receptors (mGluRs) by examining their effects on the signal transduction of mGluR1, mGluR2 and mGluR6 subtypes expressed in Chinese hamster ovary cells. This analysis revealed several structural characteristics that govern receptor subtype specificity of the agonist and antagonist activities of phenylglycine derivatives. 2. Hydroxyphenylglycine derivatives possessed either an agonist activity on mGluR1/mGluR6 or an antagonist activity on mGluR1. 3. Carboxyphenylglycine derivatives showed an agonist activity on mGluR2 but an antagonist activity on mGluR1. 4. alpha-Methylation or alpha-ethylation of the carboxyphenylglycine derivatives converts the agonist property for mGluR2 to an antagonist property, thus producing antagonists at both mGluR1 and mGluR2. 5. Structurally-corresponding phenylalanine derivatives showed little or no agonist or antagonist activity on any subtypes of the receptors. 6. This investigation demonstrates that the nature and positions of side chains and ring substituents incorporated into the phenylglycine structure are critical in determining the agonist and antagonist activities of members of this group of compounds on different subtypes of the mGluR family. 7. We also tested two alpha-methyl derivatives of mGluR agonists. (2S, 1'S, 2'S)-2-(2-Carboxycyclopropyl)glycine (L-CCG-I) is a potent agonist for mGluR2 but alpha-methylation of this compound changes its activity to that of an mGluR2-selective antagonist. In contrast, alpha-methylation of L-2-amino-4-phosphonobutyrate (L-AP4) results in retention of an agonist activity on mGluR6. Thus, alpha-methylation produces different effects, depending on the chemical structures of lead compounds and/or on the subtype of mGluR tested.
Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes.[Pubmed:8182479]
J Neurosci. 1994 May;14(5 Pt 2):3370-7.
The metabotropic glutamate receptors (mGluRs) consist of at least seven different subtypes and are coupled to intracellular signal transduction via G proteins. However, the lack of specific antagonists for the mGluRs limited the precise characterization of the role of the individual mGluRs. In this study, we investigated the agonist and antagonist activities of a series of phenylglycine derivatives for the mGluRs by examining their effects on the signal transduction of representative mGluR1, mGluR2, and mGluR4 subtypes expressed individually in Chinese hamster ovary cells. The phenylglycine derivatives examined included (S)- and (R)-forms of 3-hydroxyphenylglycine (3HPG), 4-carboxy-phenylglycine (4CPG), 4-carboxy-3-hydroxyphenylglycine (4C3HPG), 3-carboxy-4-hydroxyphenylglycine (3C4HPG), and (+)- and (-)-alpha-methyl-4-carboxyphenylglycine (alpha M4CPG). Among these 10 compounds, (S)-3HPG acted as an agonist for mGluR1, while (S)-4C3HPG, (S)-3C4HPG, and (S)-4CPG served as effective agonists for mGluR2. The rank order of agonist potencies for mGluR2 was L-glutamate > (S)-4C3HPG > (S)-3C4HPG > (S)-4CPG. No other phenylglycine derivatives showed any definite agonist activity on either mGluR1 or mGluR2. Among the phenylglycine derivatives with no mGluR1 agonist activity, (S)-4C3HPG, (S)-3C4HPG, (S)-4CPG, and (+)-alpha M4CPG effectively antagonized the action of L-glutamate on mGluR1. The rank order of antagonist potencies was (S)-4C3HPG > or = (S)-4CPG > or = (+)-alpha M4CPG > (S)-3C4HPG. The Schild plot analysis indicated that (RS)-4C3HPG, (S)-4CPG, and (+)-alpha M4CPG all act as competitive antagonists for mGluR1 with pA2 values of 4.38, 4.46, and 4.38, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Phenylglycine derivatives as antagonists of metabotropic glutamate receptors.[Pubmed:7992387]
Trends Pharmacol Sci. 1994 Sep;15(9):333-42.
Metabotropic glutamate receptors represent a family of G protein-coupled receptors that can be activated by L-glutamate, the principal excitatory neurotransmitter in the brain. Until recently, progress in identifying the physiological and pathological roles of metabotropic glutamate receptors has been hampered by the lack of selective antagonists. In this article, Jeff Watkins and Graham Collingridge describe the pharmacology of, and initial physiological studies using, certain phenylglycine derivatives and related substances--the first definitive antagonists of metabotropic glutamate receptors.


