(R)-CPPCAS# 126453-07-4 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 126453-07-4 | SDF | Download SDF |
| PubChem ID | 6603754 | Appearance | Powder |
| Formula | C8H17N2O5P | M.Wt | 252.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
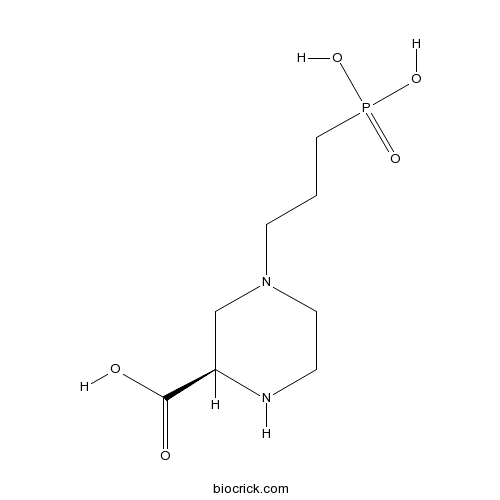
| Chemical Name | (2R)-4-(3-phosphonopropyl)piperazine-2-carboxylic acid | ||
| SMILES | C1CN(CC(N1)C(=O)O)CCCP(=O)(O)O | ||
| Standard InChIKey | CUVGUPIVTLGRGI-SSDOTTSWSA-N | ||
| Standard InChI | InChI=1S/C8H17N2O5P/c11-8(12)7-6-10(4-2-9-7)3-1-5-16(13,14)15/h7,9H,1-6H2,(H,11,12)(H2,13,14,15)/t7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent NMDA antagonist; more active isomer. Shows some selectivity for NR2A-containing receptors (Ki values are 0.041, 0.27, 0.63 and 1.99 μM for inhibition of NR2A-, NR2B-, NR2C- and NR2D-containing recombinant NMDA receptors respectively). (RS)-CPP also available. |

(R)-CPP Dilution Calculator

(R)-CPP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9649 mL | 19.8247 mL | 39.6495 mL | 79.299 mL | 99.1237 mL |
| 5 mM | 0.793 mL | 3.9649 mL | 7.9299 mL | 15.8598 mL | 19.8247 mL |
| 10 mM | 0.3965 mL | 1.9825 mL | 3.9649 mL | 7.9299 mL | 9.9124 mL |
| 50 mM | 0.0793 mL | 0.3965 mL | 0.793 mL | 1.586 mL | 1.9825 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3965 mL | 0.793 mL | 0.9912 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Ethoxygeniposide
Catalog No.:BCN7043
CAS No.:1264496-61-8
- Pyrrolam B
Catalog No.:BCN1986
CAS No.:126424-77-9
- Pyrrolam A
Catalog No.:BCN2040
CAS No.:126424-76-8
- VTP-27999 Hydrochloride
Catalog No.:BCC2050
CAS No.:1264191-73-2
- SR 12813
Catalog No.:BCC7530
CAS No.:126411-39-0
- Colistin Sulfate
Catalog No.:BCC4653
CAS No.:1264-72-8
- Nortropinyl cinnamate
Catalog No.:BCN1891
CAS No.:126394-79-4
- Pinobanksin 3-O-propanoate
Catalog No.:BCN7737
CAS No.:126394-70-5
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- DL-AP4 Sodium salt
Catalog No.:BCC7759
CAS No.:1263093-79-3
- Paromomycin Sulfate
Catalog No.:BCC4694
CAS No.:1263-89-4
- (1S,2R)-1-Amino-2-indanol
Catalog No.:BCC8384
CAS No.:126456-43-7
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
Stereoselective enhancement by (R)-HA-966 of the binding of [3H]CPP to the NMDA receptor complex.[Pubmed:2147657]
Eur J Pharmacol. 1990 Sep 18;189(2-3):237-40.
The enantiomers of the strychnine-insensitive glycine antagonist, HA-966 (1-hydroxy-3-amino-pyrrolidone-2), stereoselectively enhance binding of the N-methyl-D-aspartate (NMDA) competitive antagonist, [3H]CPP (3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid) to rat brain synaptosomal membranes. The enhancement by the more potent (R)-HA-966 is competitively inhibited by the glycine antagonist 7-chlorokynurenic acid and noncompetitively by the polyamine spermine. Thus, (R)-HA-966, apparently at the glycine site, enhances the binding of antagonist to the NMDA receptor, possibly through a mechanism partially in common with that of spermine.
Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid.[Pubmed:14718249]
Br J Pharmacol. 2004 Feb;141(3):508-16.
(2S*,3R*)-1-(biphenyl-4-carbonyl)piperazine-2,3-dicarboxylic acid (PBPD) is a moderate affinity, competitive N-methyl-d-aspartate (NMDA) receptor antagonist with an atypical pattern of selectivity among NMDA receptor 2 subunit (NR2) subunits. We now describe the activity of several derivatives of PBPD tested at both rat brain NMDA receptors using l-[3H]-glutamate binding assays and at recombinant receptors expressed in Xenopus oocytes. Substituting various branched ring structures for the biphenyl group of PBPD reduced NMDA receptor activity. However, substituting linearly arranged ring structures - fluorenone or phenanthrene groups - retained or enhanced activity. Relative to PBPD, the phenanthrene derivative (2S*, 3R*)-1-(phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA) displayed a 30- to 78-fold increase in affinity for native NMDA receptors. At recombinant receptors, PPDA displayed a 16-fold (NR2B) to 94-fold (NR2C) increase in affinity over PBPD. Replacement of the biphenyl group of PBPD with a 9-oxofluorene ring system resulted in small changes in receptor affinity and subtype selectivity. 2'-Bromo substitution on the biphenyl group of PBPD reduced antagonist affinity 3- to 5-fold at NR2A-, NR2B- and NR2D-containing receptors, but had little effect on NR2C-containing receptors. In contrast, 4'-fluoro substitution of the biphenyl ring of PBPD selectively increased NR2A affinity. The aromatic rings of PBPD and PPDA increase antagonist affinity and appear to interact with a region of the NMDA receptor displaying subunit heterogeneity. PPDA is the most potent and selective NR2C/NR2D-preferring antagonist yet reported and thus may be useful in defining NR2C/NR2D function and developing related antagonists with improved NMDA receptor subtype selectivity. British Journal of Pharmacology (2004) 141, 508-516. doi:10.1038/sj.bjp.0705644


