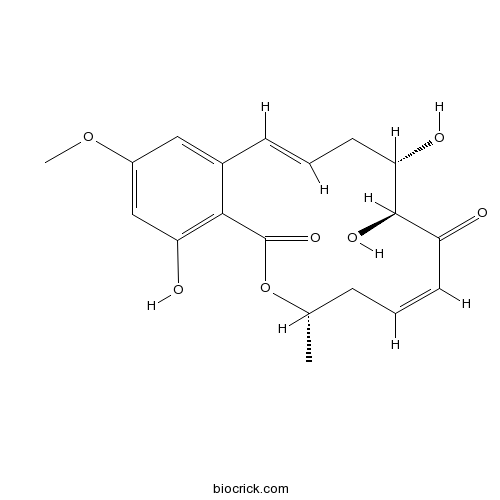
(5Z)-7-OxozeaenolPotent and selective TAK1 MAPKKK inhibitor CAS# 66018-38-0 |
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Rivaroxaban
Catalog No.:BCC2292
CAS No.:366789-02-8
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 66018-38-0 | SDF | Download SDF |
| PubChem ID | 9863776 | Appearance | Powder |
| Formula | C19H22O7 | M.Wt | 362.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO and to 10 mM in ethanol | ||
| Chemical Name | (4S,6Z,9S,10S,12E)-9,10,18-trihydroxy-16-methoxy-4-methyl-3-oxabicyclo[12.4.0]octadeca-1(14),6,12,15,17-pentaene-2,8-dione | ||
| SMILES | CC1CC=CC(=O)C(C(CC=CC2=CC(=CC(=C2C(=O)O1)O)OC)O)O | ||
| Standard InChIKey | NEQZWEXWOFPKOT-BYRRXHGESA-N | ||
| Standard InChI | InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h3-4,6-7,9-11,15,18,21-23H,5,8H2,1-2H3/b6-4+,7-3-/t11-,15-,18+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Resorcyclic lactone of fungal origin that acts as a potent and selective transforming growth factor-β-activated kinase 1 (TAK1) mitogen-activated protein kinase kinase kinase (MAPKKK) inhibitor (IC50 = 8 nM). Displays > 33-fold and > 62-fold selectivity over MEKK1 and MEKK4 respectively. Inhibits IL-1-induced activation of NF-κB (IC50 = 83 nM) and JNK/p38. Inhibits production of inflammatory mediators, and sensitizes cells to TRAIL- and TNF-α-induced apoptosis in vitro. |

(5Z)-7-Oxozeaenol Dilution Calculator

(5Z)-7-Oxozeaenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7596 mL | 13.7981 mL | 27.5961 mL | 55.1922 mL | 68.9903 mL |
| 5 mM | 0.5519 mL | 2.7596 mL | 5.5192 mL | 11.0384 mL | 13.7981 mL |
| 10 mM | 0.276 mL | 1.3798 mL | 2.7596 mL | 5.5192 mL | 6.899 mL |
| 50 mM | 0.0552 mL | 0.276 mL | 0.5519 mL | 1.1038 mL | 1.3798 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.276 mL | 0.5519 mL | 0.6899 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Demethoxytangeretin
Catalog No.:BCN3844
CAS No.:6601-66-7
- Cirsimaritin
Catalog No.:BCN4206
CAS No.:6601-62-3
- H-Nva-OH
Catalog No.:BCC2643
CAS No.:6600-40-4
- Psoralen
Catalog No.:BCN4219
CAS No.:66-97-7
- D-Glucosamine hydrochloride
Catalog No.:BCN5982
CAS No.:66-84-2
- Cycloheximide
Catalog No.:BCC3653
CAS No.:66-81-9
- Dicoumarol
Catalog No.:BCC9225
CAS No.:66-76-2
- Strophantidin
Catalog No.:BCC8255
CAS No.:66-28-4
- Uracil
Catalog No.:BCN4211
CAS No.:66-22-8
- Punicalin
Catalog No.:BCN4961
CAS No.:65995-64-4
- Punicalagin
Catalog No.:BCN1037
CAS No.:65995-63-3
- Isogarciniaxanthone E
Catalog No.:BCN4205
CAS No.:659747-28-1
- H-D-Arg(NO2)-OH
Catalog No.:BCC2871
CAS No.:66036-77-9
- Glycyrin
Catalog No.:BCN7681
CAS No.:66056-18-6
- Licoisoflavone A
Catalog No.:BCN2929
CAS No.:66056-19-7
- Gomisin H
Catalog No.:BCN3902
CAS No.:66056-20-0
- Angeloylgomisin H
Catalog No.:BCN2843
CAS No.:66056-22-2
- Benzoylgomisin H
Catalog No.:BCN7242
CAS No.:66056-23-3
- Licoisoflavone B
Catalog No.:BCN6695
CAS No.:66056-30-2
- Licoisoflavanone
Catalog No.:BCN6856
CAS No.:66067-26-3
- Tigloylgomisin H
Catalog No.:BCN6927
CAS No.:66069-55-4
- GW 542573X
Catalog No.:BCC7914
CAS No.:660846-41-3
- Nimodipine
Catalog No.:BCC3823
CAS No.:66085-59-4
- GW843682X
Catalog No.:BCC1614
CAS No.:660868-91-7
Enhancement of hyperthermia-induced apoptosis by 5Z-7-oxozeaenol, a TAK1 inhibitor, in A549 cells.[Pubmed:27448221]
Cell Stress Chaperones. 2016 Sep;21(5):873-81.
KRAS mutant lung cancers have long been considered as untreatable with drugs. Transforming growth factor-beta-activated kinase 1 (TAK1) appears to play an anti-apoptotic role in response to multiple stresses and has been reported to be a responsive kinase that regulates cell survival in KRAS-dependent cells. In this study, in order to find a useful approach to treat KRAS mutant lung cancer, we focused on the combined effects of 5Z-7-oxozeaenol, a TAK1 inhibitor, with hyperthermia (HT) in KRAS mutant lung cancer cell line A549. Annexin V-FITC/PI assay, cell cycle analysis, and colony formation assay revealed a significant enhancement in apoptosis induced by HT treatment, when the cells were pre-incubated with 5Z-7-oxozeaenol in a dose-dependent manner. The enhanced apoptosis by 5Z-7-oxozeaenol was accompanied by a significant increase in reactive oxygen species (ROS) generation and loss of mitochondrial membrane potential (MMP). In addition, western blot showed that 5Z-7-oxozeaenol enhanced HT-induced expressions of cleaved caspase-3, cleaved caspase-8, and HSP70 and decreased HT-induced expressions of Bcl-2, p-p38, p-JNK, and LC3. Moreover, 5Z-7-oxozeaenol pre-treatment resulted in a marked elevation of intracellular calcium level which might be associated with endoplasmic reticulum (ER) stress-related pathway. Taken together, our data provides further insights of the mechanism of action of 5Z-7-oxozeaenol and HT treatment, and their potential application as a novel approache to treat patients with KRAS mutant lung cancer.
Synergistic action of 5Z-7-oxozeaenol and bortezomib in inducing apoptosis of Burkitt lymphoma cell line Daudi.[Pubmed:26227222]
Tumour Biol. 2016 Jan;37(1):531-9.
Treatment failure in cancer chemotherapy is largely due to the toxic effects of chemotherapeutic agents on normal cells/tissues. The proteasome inhibitor bortezomib has been successfully applied to treat multiple myeloma (MM), but there are some common adverse reactions in the clinic including peripheral neuropathy (PN). The TAK1 selective inhibitor 5Z-7-oxozeaenol has been widely studied in cancer therapy. Here, we investigated the potential synergy of bortezomib and 5Z-7-oxozeaenol in Burkitt's lymphoma (BL) cell lines. Cell viability assay showed that co-treatment of bortezomib at 8 nM, representing a one-eighth concentration for growth arrest, and 5Z-7-oxozeaenol at 2 muM, a dose that exhibited insignificant cytotoxic effects, synergistically induced apoptosis in the cell line Daudi. In parallel with the increasing dose of the bortezomib, and 5Z-7-oxozeaenol at 0.5 muM, lower colony formation efficiencies were seen in the cell line Daudi. Western blotting analysis verified that TAK1 inhibition by 5Z-7-oxozeaenol completely blocked JNK, p38, Erk, IKK, and IkappaB phosphorylation, which was almost instantly activated by TAK1 both directly or indirectly. Both agents synergistically prevented nuclear translocation of NF-kappaB, a characteristic of NF-kappaB inactivation. Moreover, a synergistic effect of bortezomib and 5Z-7-oxozeaenol on Western blotting analysis and flow cytometry was disclosed. Collectively, our results indicated that the proteasome inhibitor bortezomib and the TAK1 inhibitor 5Z-7-oxozeaenol displayed synergy on inhibiting BL cell apoptosis by inhibiting NF-kappaB activity.
Enhancement of hyperthermia-induced apoptosis by 5Z-7-oxozeaenol, a TAK1 inhibitor, in Molt-4 cells.[Pubmed:28111999]
Int J Hyperthermia. 2017 Jun;33(4):411-418.
PURPOSE: Transforming growth factor-beta-activated kinase1 (TAK1) plays an anti-apoptotic role in response to multiple stresses. TAK1 inhibitor, 5Z-7-oxozeaenol (OZ) has been studied for its apoptotic effects. However, the combined effect of OZ with physical stresses remains to be elusive. Therefore, in this study we focussed to determine the combined effects of OZ with hyperthermia (HT) using Molt-4 cell line. MATERIALS AND METHODS: Molt-4 cells were pre-treated with OZ for 1 h followed by heat exposure (44 degrees C, 10 min) and harvested 24 h after incubation at 37 degrees C, apoptosis was measured by Annexin V-FITC/PI double staining assay using flow cytometry and cell growth was observed by cell counting assay. Further mechanism involved in the combination was investigated by measuring mitochondrial membrane potential (MMP), intracellular ROS generation, expression of apoptosis related protein, intracellular calcium ion level and Fas activity. RESULTS: Combination of OZ with HT significantly enhances MMP loss and superoxide generation. Furthermore, OZ pre-treatment promotes caspase-8 cleavage, Fas externalisation, caspase 3 activity and intracellular calcium ion levels. OZ pre-treatment decreased the expression of HT-induced Bcl-2 and increased the expression of pro-apoptotic Bax, while markedly suppressed the phosphorylation of JNK and p38. In addition, increased expression of CHOP following combined treatment indicates that ER stress may also involve in the enhancement of HT-induced apoptosis. CONCLUSION: Our data showed for the first time that OZ sensitizes Molt-4 cells to HT-induced apoptosis via extrinsic and intrinsic apoptotic pathways. Furthermore, ROS and ER stress may also play role in the enhancement of HT-induced apoptosis by OZ.
Isolation, semisynthesis, covalent docking and transforming growth factor beta-activated kinase 1 (TAK1)-inhibitory activities of (5Z)-7-oxozeaenol analogues.[Pubmed:26481152]
Bioorg Med Chem. 2015 Nov 1;23(21):6993-9.
(5Z)-7-Oxozeanol and related analogues were isolated and screened to explore their activity as TAK1 inhibitors. Seven analogues were synthesized and more than a score of natural products isolated that examined the role that different areas of the molecule contribute to TAK1 inhibition. A novel nonaromatic difluoro-derivative was synthesized that had similar potency compared to the lead. This is the first example of a nonaromatic compound in this class to have TAK1 inhibition. Covalent docking for the isolated and synthesized analogues was carried out and found a strong correlation between the observed activities and the calculated binding.
Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide.[Pubmed:17348859]
Biochem J. 2007 Jun 1;404(2):179-90.
MDP (muramyl dipeptide), a component of peptidoglycan, interacts with NOD2 (nucleotide-binding oligomerization domain 2) stimulating the NOD2-RIP2 (receptor-interacting protein 2) complex to activate signalling pathways important for antibacterial defence. Here we demonstrate that the protein kinase activity of RIP2 has two functions, namely to limit the strength of downstream signalling and to stabilize the active enzyme. Thus pharmacological inhibition of RIP2 kinase with either SB 203580 [a p38 MAPK (mitogen-activated protein kinase) inhibitor] or the Src family kinase inhibitor PP2 induces a rapid and drastic decrease in the level of the RIP2 protein, which may explain why these RIP2 inhibitors block MDP-stimulated downstream signalling and the production of IL-1beta (interleukin-1beta) and TNFalpha (tumour necrosis factor-alpha). We also show that RIP2 induces the activation of the protein kinase TAK1 (transforming-growth-factor-beta-activated kinase-1), that a dominant-negative mutant of TAK1 inhibits RIP2-induced activation of JNK (c-Jun N-terminal kinase) and p38alpha MAPK, and that signalling downstream of NOD2 or RIP2 is reduced by the TAK1 inhibitor (5Z)-7-Oxozeaenol or in TAK1-deficient cells. We also show that MDP activates ERK1 (extracellular-signal-regulated kinase 1)/ERK2 and p38alpha MAPK in human peripheral-blood mononuclear cells and that the activity of both MAPKs and TAK1 are required for MDP-induced signalling and production of IL-1beta and TNFalpha in these cells. Taken together, our results indicate that the MDP-NOD2/RIP2 and LPS (lipopolysaccharide)-TLR4 (Toll-like receptor 4) signalling pathways converge at the level of TAK1 and that many subsequent events that lead to the production of pro-inflammatory cytokines are common to both pathways.
Blockade of transforming growth factor-beta-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade.[Pubmed:17172402]
Mol Cancer Ther. 2006 Dec;5(12):2970-6.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL/Apo2L) is a member of the TNF-alpha ligand family that selectively induces apoptosis in a variety of tumor cells. To clarify the molecular mechanism of TRAIL-induced apoptosis, we focused on transforming growth factor-beta-activated kinase 1 (TAK1) mitogen-activated protein kinase (MAPK) kinase kinase, a key regulator of the TNF-alpha-induced activation of p65/RelA and c-Jun NH2-terminal kinase/p38 MAPKs. In human cervical carcinoma HeLa cells, TRAIL induced the delayed phosphorylation of endogenous TAK1 and its activator protein TAB1 and TAB2, which contrasted to the rapid response to TNF-alpha. Specific knockdown of TAK1 using small interfering RNA (siRNA) abrogated the TRAIL-induced activation of p65 and c-Jun NH2-terminal kinase/p38 MAPKs. TRAIL-induced apoptotic signals, including caspase-8, caspase-3, caspase-7, and poly(ADP-ribose) polymerase, were enhanced by TAK1 siRNA. Flow cytometry showed that the binding of Annexin V to cell surface was also synergistically increased by TRAIL in combination with TAK1 siRNA. In addition, pretreatment of cells with 5Z-7-oxozeaenol, a selective TAK1 kinase inhibitor, enhanced the TRAIL-induced cleavage of caspases and binding of Annexin V. The TAK1-mediated antiapoptotic effects were also observed in human lung adenocarcinoma A549 cells. In contrast, TAK1-deficient mouse embryonic fibroblasts are resistant to TRAIL-induced apoptosis, and treatment of control mouse embryonic fibroblasts with 5Z-7-oxozeaenol did not drastically promote the TRAIL-induced activation of a caspase cascade. These results suggest that TAK1 plays a critical role for TRAIL-induced apoptosis, and the blockade of TAK1 kinase will improve the chances of overcoming cancer.
A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase.[Pubmed:12624112]
J Biol Chem. 2003 May 16;278(20):18485-90.
TAK1, a member of the mitogen-activated kinase kinase kinase (MAPKKK) family, participates in proinflammatory cellular signaling pathways by activating JNK/p38 MAPKs and NF-kappaB. To identify drugs that prevent inflammation, we screened inhibitors of TAK1 catalytic activity. We identified a natural resorcylic lactone of fungal origin, 5Z-7-oxozeaenol, as a highly potent inhibitor of TAK1. This compound did not effectively inhibit the catalytic activities of the MEKK1 or ASK1 MAPKKKs, suggesting that 5Z-7-oxozeaenol is a selective inhibitor of TAK1. In cell culture, 5Z-7-oxozeaenol blocked interleukin-1-induced activation of TAK1, JNK/p38 MAPK, IkappaB kinases, and NF-kappaB, resulting in inhibition of cyclooxgenase-2 production. Furthermore, in vivo 5Z-7-oxozeaenol was able to inhibit picryl chloride-induced ear swelling. Thus, 5Z-7-oxozeaenol blocks proinflammatory signaling by selectively inhibiting TAK1 MAPKKK.


